Background and overview[1][2][3]
4-Aminobenzaldehyde is a light yellow crystal, used in the synthesis of sulfonamide antibacterial synergist trimethoprim (TMP); used in the spice industry as an intermediate for anisaldehyde and vanillin; in the pesticide industry used for The raw material of chlorobenzaldehyde can also be used for the manufacture of photoresistors and the synthesis of mechanical metal protective films. Most of the existing synthesis methods use p-nitrotoluene through oxidation and reduction. Dissolve sodium sulfide and sodium hydroxide in water, filter, heat the filtrate, add sulfur powder, add ethanol and p-nitrotoluene, and recover ethanol after the reaction. Steam distillation removes the by-product p-aminotoluene, and the reaction solution is extracted with benzene, followed by steam distillation, cooling, filtering, and drying to obtain the finished product.
This synthesis method requires the use of sodium sulfide, sodium hydroxide, and sulfur powder as reaction raw materials. The reaction raw material sodium sulfide deliquesces in the air and gradually undergoes oxidation; it can explode upon impact and high heat, and can produce toxic hydrogen sulfide when exposed to acid. The gas is flammable and emits toxic sulfur oxide fumes when heated. These factors will increase the risk factor of the reaction process and endanger the health of synthesis operators.
The reaction raw material sodium hydroxide is highly irritating and corrosive. Dust or smoke will irritate the eyes and respiratory tract, corrode the nasal septum, and cause greater health hazards to production operators; and the strong corrosiveness of sodium hydroxide will increase the corrosion resistance requirements of the reaction device. , leading to an increase in the manufacturing cost of the reaction device, which is not conducive to reducing the reaction cost.
The reaction raw material sulfur powder is flammable and explosive. It is easy to explode or burn when mixed with air. The steam and sulfur dioxide produced after burning sulfur are highly toxic to the human body. Violent reactions will occur when in contact with halogens, metal powders, etc. , Sulfur is a poor conductor. It will generate static charges during storage and transportation, and can also cause sulfur dust to catch fire. When the dust is mixed with air or oxidants, an explosive mixture will be formed. These factors will increase the risk factor of the synthesis reaction process and are not conducive to safety. Production.
Apply[3]
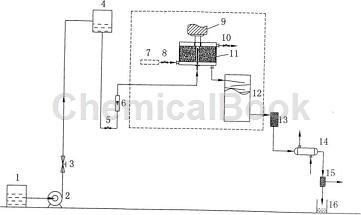
Preparation of p-hydroxybenzaldehyde by hydrolysis of diazonium salt of p-aminobenzaldehyde: Pump the prepared diazonium salt solution of p-aminobenzaldehyde into the high tank 4 with pump 2, and adjust the rotating speed of the rotating packed bed 11 to 600r/ min~2000r/min, adjust the opening of the discharge valve 5 of the high-level tank 4, and enter the rotating packing bed 11 at a flow rate of 0.5m3/h~3.0m3/h through the flowmeter 6. At the same time, open the heat medium air inlet valve 8. Appropriately open the heat medium outlet valve 10 so that the diazonium salt solution is quickly and evenly heated to 65°C to 95°C in the rotating packed bed, undergoes a rapid hydrolysis reaction, and then enters the guide plate tower at the bottom of the rotating packed bed 11 12 Carry out subsequent hydrolysis reaction.
The total residence time of the reaction material through the rotating packed bed 11 and the guide plate tower 12 is 60s to 180s, and then the reaction liquid is filtered through the filter 113 to remove the oily by-product, and then enters the tubular condenser 14 for hydrolysis The liquid is quickly cooled to room temperature. After cooling, the main product crystallizes out from the water and is filtered with filter II 15. The filter cake is the product p-hydroxybenzaldehyde containing a certain amount of moisture. The filtrate is a saturated aqueous solution (mother liquor) of p-hydroxybenzaldehyde, which is recovered. The mother liquor is sent to the mother liquor recovery tank 16, and the resulting product (filter cake) is washed with ice water several times, and dried in vacuum to a constant weight to obtain p-hydroxybenzaldehyde, the main product of hydrolysis. The final product yield is 65% to 83%.
Preparation[2]
Method 1: Oxidation and reduction of p-nitrobenzyl alcohol to prepare 4-aminobenzaldehyde
A: Add 2 mol of p-nitrobenzyl alcohol and 1.3 L of 10% potassium sulfate solution to the reaction vessel. Control the solution temperature to 30°C, add 4 mol of lead dioxide, control the stirring speed to 310 rpm, and continue the reaction for 70 minutes;
B: Then add 4 mol of 15% cyclohexane solution, add 4 mol of nickel chloride powder three times within 30 minutes, raise the temperature of the solution to 50°C, react for 3 hours, lower the temperature to 5°C, and let stand. Leave it for 30 minutes, add a mass fraction of 10% sodium chloride solution and wash for 30 minutes, a mass fraction of 30% diethylene glycol monoethyl ether solution for 40 minutes, a mass fraction of 40% butyl ether solution for 60 minutes, and add a mass fraction of 60% cyclooctane. Recrystallize in the solution and dehydrate with anhydrous magnesium sulfate dehydrating agent to obtain 237.402g of the finished product 4-aminobenzaldehyde, with a yield of 98.1%.

Method 2: Method for preparing 4-aminobenzaldehyde by catalytic hydrogenation of p-nitrobenzaldehyde
Nitro compounds are reduced to amino compounds. They were first prepared by the method of iron powder and hydrochloric acid. This method is seriously polluting and is a process route that the country has explicitly stipulated will be abolished in the near future. In recent years, most of this type of production uses thunder. Nickel catalyst, that is, using Raney nickel as a catalyst to achieve the conversion from nitro to amino group through catalytic hydrogenation, but using Raney nickel catalyst for catalytic hydrogenation has the following disadvantages:
1) It is extremely inconvenient to use. Because the active ingredient of Raney nickel catalyst for catalytic hydrogenation is skeleton nickel, but skeleton nickel easily catches fire in the air and cannot be stored. It can only be commercialized in the form of nickel-aluminum alloy powder, which needs to be treated with alkali before use. The aluminum is dissolved, washed and added to the reaction system under air-isolated conditions; in addition, the catalytic activity of Raney nickel often changes greatly due to different treatment conditions (such as alkali dissolution and washing conditions).
2) When Raney nickel catalyst is used, the amount of reaction by-products is large and the product yield is low. Hydrogenation of Raney nickel catalyst to convert nitro group into amino group often requires higher temperature, generally the required temperature is higher than 100°C, and the amino compounds generated by hydrogenation are easy to produce by-products at temperatures higher than 100°C ( It is called tar in industry. On the one hand, it reduces the product yield, and on the other hand, it may affect the normal progress of the reaction.
3) The catalyst consumption is large. Due to the low catalytic activity of Raney Nickel, a large amount of catalyst needs to be added. On the other hand, the recovery of the catalyst is extremely difficult, resulting in excessive catalyst consumption and high production costs.
4) There are huge safety risks in production. Because Raney nickel is easy to catch fire when exposed to air, a fire may occur if you are not careful during operation; at the same time, there is hydrogen in the hydrogenation workshop, which is prone to explosion. The following method is to improve the preparation process.
First add 100 grams of p-nitrobenzaldehyde to the reaction kettle, then add 95% ethanol with a concentration of 4 times the mass of p-nitrobenzaldehyde, and then add 10 to 15% of the mass of p-nitrobenzaldehyde. Supported catalyst, sealed reactor, completely replace the air in the reactor with hydrogen, then keep the reactor at normal pressure (about 0.1MPa), and start heating and stirring. After the temperature reaches 60°C, hydrogen is introduced into the reactor to make the reactor The pressure is maintained at 1.0~3.0MPa (tests show that the optimal pressure should be about 1.5MPa), and the hydrogenation reaction is started. During the hydrogenation process, the temperature in the kettle is maintained in the range of 70~95°C, and the hydrogenation takes about 0.5 to 1.5 hours. The final conversion rate reaches 88-90%.
Then the reaction mixture and catalyst are separated, and the separated catalyst is returned to the reactor for continued use, and the lost amount of catalyst is replenished. The test shows that only about 100 mg of catalyst is needed each time; for the reaction mixture, that is, the clear liquid The reaction product was separated to obtain 70.5 to 72.1 grams of 4-aminobenzaldehyde. In the present invention, the specific method adopted is to first transfer the reaction system into an insulated settling tank to separate the catalyst and the reaction mixture.
The test shows that during the above reaction process, the amount of supported catalyst added is 13% of the mass of p-nitrobenzaldehyde added, the pressure in the kettle during hydrogenation is 1.5Mpa, and the reaction temperature is 90°C. The conversion rate reaches over 90.0% within 1 hour.
Main reference materials
[1]Ma Hongfei. (1997). Research on new synthesis methods of p-aminobenzaldehyde. Journal of Yancheng Institute of Technology (Natural Science Edition) (4), 35-38.
[2]Weng Yuankai, & Huang Shan. (1992). Kinetics of synthesis of p-aminobenzaldehyde by disproportionation reaction. Journal of China Pharmaceutical University (4), 33-35.
[3]Xu Zhishi. (2000). Improvement of the method for preparing p-aminobenzaldehyde using sodium polysulfide. Journal of Jilin Radio and Television University (3), 61-62.

 微信扫一扫打赏
微信扫一扫打赏

