Background and overview[1][2]
Benzonitrile, also known as “cyanobenzene”, is a liquid with the smell of bitter almond volatile oil. Miscible with ethanol and ether, soluble in acetone and benzene, 1g dissolves in 100ml water at 100℃. Benzonitrile has a wide range of applications. Its greatest use is that benzogyanamide made by reacting with dicyandiamide can be used in metal coatings, plastics, fluorescent pigments, molding materials, etc. Benzonitrile can also be used in organic synthesis, paints, High boiling point solvent for printing inks and resins, as well as as an additive in jet fuel and as a combustion accelerant for synthetic fibers. Market demand is increasing year by year. The reactions involved are mainly cyano reactions:
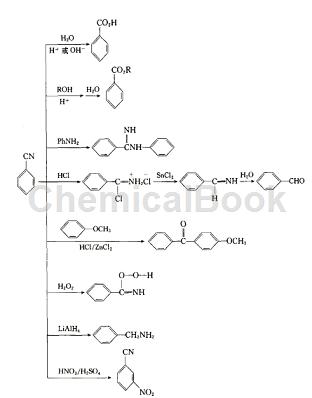
Preparation[2]
According to foreign information, Japan was the first to realize the industrialization of aromatic nitriles produced by toluene ammoxidation. In 1963, Nitto Chemical Industry Company used a 2,6-dichlorotoluene ammoxidation method to produce 2,6-dichlorobenzonitrile based on a self-developed catalyst and fluidized bed reaction process. In 1969, Nippon Shokubai Chemical Industry Co., Ltd. used the ammoxidation method of mixed aromatic hydrocarbons to produce phthalonitrile and benzonitrile. In 1970, Showa Denko (SDK) of Japan and Diamond Shamrock of the United States used SDK ammonia oxidation technology to produce isophthalonitrile in Yokohama, Japan. In order to make full use of m-xylene resources, Japan’s Mitsubishi Gas Chemical Company cooperated with the American Bagder Company to produce isophthalonitrile using a fluidized bed process with fine particle catalysts. During this period, Tokyo Organic Company in Japan also adopted an industrial production method of 3-methylpyridine ammonia oxidation to produce 3-cyanopyridine. Since then, aromatic hydrocarbon ammoxidation technology has been widely used in the synthesis of fine chemicals such as aromatic nitriles, and has become the most important method for manufacturing aromatic nitriles.
Currently, domestic production plants also use the toluene ammoxidation method to produce benzonitrile, which generally has the following shortcomings:
1. Production plants generally use coarse particle catalyst fluidization technology. The carrier of the coarse particle catalyst is alumina, and the active components are mainly metal vanadium and chromium. The sieving range is 40-120 mesh, and the specific surface is Smaller than pore volume and poor mechanical strength. The coarse particle catalyst itself has poor activity, selectivity and stability, resulting in low conversion rate and yield. Moreover, the catalyst has a short service life and loses activity in a short period of time, so the catalyst must be removed and regenerated.
2. The contact between the reaction material gas and the solid catalyst in the reactor is uneven. Large bubbles generated by the coarse particle catalyst often cause channeling, throttling and other phenomena, which affect the fluidization quality. Moreover, the material gas stays in the bed for too long, triggering secondary reactions and exacerbating the deterioration of the process.
3. The distillation method adopts normal pressure distillation and the temperature is relatively high, so that the product purity has been hovering between 98% and 99% and cannot be improved.
The above-mentioned production process has low product yield, high material and energy consumption, serious environmental pollution, and product purity cannot meet user requirements. CN200710039927 provides a production method of benzonitrile, which also uses toluene, ammonia and air as raw materials, and uses a toluene ammoxidation method under the action of a catalyst to produce, using a fluidized bed process of fine particle catalysts. The reaction equation is as follows:

The production method of benzonitrile of the present invention uses a fine particle catalyst with high activity and good selectivity, and selects appropriate reaction temperature, appropriate reaction material gas flow and other process conditions to make it more conducive to the reaction and product quality. improve.
The production method of benzonitrile of the present invention includes the following steps:
(1) Toluene ammoxidation reaction
The toluene and liquid ammonia materials are heated separately, vaporized, and mixed before entering the fluidized bed reactor, where they come into contact with the hot air and react under the action of the fine particle catalyst BN-98. The flow rate of toluene is 90-110l/h, the flow rate of liquid ammonia is 60-80m3/h, the flow rate of air is 300-500m3/h, and the reaction temperature is 410 -420℃, the catalyst load is 37-45g toluene/liter catalyst·hour, the material preheating temperature is 190-200℃, the material gas pressure entering the reactor is 0.03-0.05MPa, and continuous production is adopted;
(2) Condensation and separation to obtain crude benzonitrile
The temperature of the product gas flowing out from the fluidized bed reactor can reach about 300°C. The air can be preheated through heat exchange to save energy, and then enter the capture tower and absorption tower for spray water cooling , condensed into liquid phase benzonitrile and water, the upper layer benzonitrile enters the separator through overflow, and is separated into crude benzonitrile. The production wastewater separated from the lower layer can be used as a combined spray for the capture tower and absorption tower. Water, recycled use, saving water resources;
(3) Distill under reduced pressure to obtain pure benzonitrile product
Send the separated crude benzonitrile into a distillation kettle for vacuum distillation with a vacuum degree of 0.090-0.092MPa and a heating temperature of 138-144°C to obtain a pure benzonitrile product with a content of 99.6-99.8%.
Apply [3-4]
1. Used to prepare a high-voltage electrolyte containing benzonitrile additive
CN201810259219.1 provides a high-voltage electrolyte containing benzonitrile additives and a preparation method thereof, which forms a dense and uniform protective film with low impedance on the surface of the cathode material, with structural stability, reliable effect, simple method, and simple operation. The addition amount is small, practical and economical. The present invention provides the following technical solution: a benzonitrile-containing additiveThe high-voltage electrolyte of the agent includes the following raw materials: cyclic carbonate, linear carbonate diethyl carbonate, ethyl methyl carbonate, inorganic conductive lithium salt and additive benzonitrile, in the following mass parts ratio: cyclic 1-3 parts of carbonate, 1-2 parts of linear carbonate diethyl carbonate, 1-5 parts of ethyl methyl carbonate, 1 part of inorganic conductive lithium salt and 1 part of additive benzonitrile. The preparation method includes the following steps:
S1: Mix cyclic carbonate and linear carbonate in the required ratio in a glove box containing argon, and remove water and impurities;
S2: In the glove box, add the dry inorganic conductive lithium salt to the above solvent system, stir at a suitable temperature for a certain period of time to dissolve, and obtain the basic electrolyte;
S3: Remove water and impurities from the functional additive benzonitrile;
S4: Add the processed additive benzonitrile to the basic electrolyte to obtain a high-voltage electrolyte containing the benzonitrile additive.
Compared with the existing technology, the beneficial effects of the present invention are: the high-voltage electrolyte containing benzonitrile additive and its preparation method improves the high-voltage performance by adding benzonitrile additive, and can be used during the battery cycle. A dense, uniform and low-impedance protective film is formed on the surface of the cathode material. The protective film can inhibit the oxidative decomposition of the electrolyte solvent and maintain the structural stability of the cathode material. The effect is reliable. Adding high-voltage functional additive benzonitrile improves lithium under high voltage. The ion battery stability method is simple, the operation is simple, the amount of addition is small, and it is practical and economical.
2. Preparation of benzylamine by catalytic hydrogenation of benzonitrile
Benzylamine is mainly used in the rubber and plastics industries. As the global demand for rubber and plastics increases, so does the amount of rubber and plastic curing agents consumed each year. According to incomplete statistics, my country’s current annual consumption of epoxy resin is about 100,000 tons, requiring about 25,000 tons of various curing agents. By the 10th Five-Year Plan period, the annual consumption of epoxy resin will be more than 120,000 tons, requiring curing agents. 30,000-35,000 tons, of which 65%-70% are amine curing agents, so benzylamine has a broad market. CN201410785310.9 provides a new method for preparing benzylamine through catalytic hydrogenation of benzonitrile. This method has the advantages of higher activity, benzonitrile conversion rate and benzylamine selectivity. In order to solve the above problems, the technical solution adopted by the present invention is as follows: a method for preparing benzylamine by catalytic hydrogenation of benzonitrile. The raw materials including benzonitrile and hydrogen enter a fixed bed reactor, contact with the catalyst, and generate benzonitrile including benzonitrile. Amine products; wherein, the catalyst includes an active component, a carrier and a cocatalyst, the active component is palladium, the carrier is alumina, magnesium oxide, silica, titanium dioxide, molecular sieve or activated carbon, and the cocatalyst is selected from Ba , Ca, Fe, P, B, Ti at least one. The catalytic activity of the catalyst of the present invention is high and stable, the conversion rate of benzonitrile and the selectivity of the product benzylamine are also significantly higher than those of existing similar catalysts, and the reaction can be carried out at lower temperatures and pressures, which is in line with energy saving and consumption reduction. At the same time, the catalyst preparation process is simple, has good repeatability, and achieves good technical effects.
Main reference materials
[1] Compound Dictionary
[2] CN200710039927.6 Production method of benzonitrile
[3] CN201810259219.1 A high-voltage electrolyte containing benzonitrile additive and its preparation method
[4] CN201410785310.9 Method for preparing benzylamine by catalytic hydrogenation of benzonitrile

 微信扫一扫打赏
微信扫一扫打赏

