Background and overview[1][2]
Phenyl formate is an important fine chemical intermediate with a wide range of uses in chemical synthesis. It can be used as a polymerization catalyst, plastic modifier, fiber treatment agent, and intermediate for medicines and pesticides. The main synthesis method of phenyl chloroformate is the phosgene method. Dissolve phenol in chloroform, introduce phosgene under cooling, and make the amount of phosgene absorbed be proportional to the amount of phenol and other substances. Add the other substances dropwise while stirring at 5-10°C. The amount of N,N-dimethylaniline. Then add cold water to dilute, separate the oil layer, and wash with dilute hydrochloric acid and water in sequence. After drying with anhydrous calcium chloride, the chloroform is recovered by distillation, and then rectified under reduced pressure to collect the 74-75°C (1.73kPa) fraction, which is phenyl chloroformate, with a yield of about 90%. The post-processing of this method is complex, a large amount of solvent needs to be recovered, a large amount of wastewater is generated, and a large amount of N,N-dimethylaniline is used as an acid binding agent, resulting in high production costs. A solvent-free production process for phenyl chloroformate is currently disclosed. Molten phenol is directly added to a small amount of catalyst, exposed to light at high temperature, and then through vacuum distillation to obtain phenyl chloroformate with a content greater than 98%. This method is post-processed. It is simple, avoids the use of a large amount of acid-binding agents and solvents, does not produce process wastewater, has low cost, and is suitable for industrial production. However, the existing technology still produces by-products, and the production efficiency is also relatively low, that is, during the production process, phenol will combine with the product phenyl chloroformate under the conditions of high temperature and acid binding agent to produce the by-product diphenyl carbonate (DPC). .
Apply[2-5]
Phenyl chloroformate is a corrosive oily liquid, insoluble in water, soluble in ethanol, ether, and easily soluble in petroleum ether. Phenyl chloroformate is an important organic synthesis intermediate and is widely used in chemical synthesis. It can be used as polymer catalyst, plastic modifier, fiber treatment agent, and intermediate for medicine and pesticide. For example, when used to prepare tert-butylcarbazate: phenyl chloroformate and tert-butanol are used as raw materials, and under the action of a solid alkali catalyst, an esterification reaction is carried out in an ionic liquid at 30~40°C. The esterification reaction After completion, add hydrazine hydrate to the esterification reaction solution, and perform a substitution reaction at 60 to 75°C. After the reaction is completed, the reaction solution is separated and purified to obtain the tert-butyl carbazate; it can also be used to prepare doxylamine impurities. , including the following steps: reacting doxylamine and phenyl chloroformate to obtain an intermediate product; hydrolyzing the intermediate product to obtain doxylamine impurity G. The preparation method of doxylamine impurity G proposed by the present invention involves the reaction of doxylamine and phenyl chloroformate to remove the methyl group on the N atom to obtain the doxylamine impurity G. The synthesis route is short and the reaction is selective. High, raw materials are easily available, product purity is high, reaction conditions are mild, economical and environmentally friendly, and easy to operate; to synthesize high-content nicosulfuron, phenyl chloroformate or diphenyl carbonate is used instead of the highly toxic ethyl chloroformate (or methyl ester ), using organic or inorganic catalysts, and using methylene chloride, dichloroethane, acetonitrile, etc. as solvents. First put N,N-dimethyl-2-aminosulfonyl-pyridinamide, 2-amino-4,6-dimethoxypyrimidine, catalyst 1 and solvent into the reaction kettle at one time, and then press Phenyl chloroformate or diphenyl carbonate and catalyst 2 are added sequentially. After the addition, the temperature is raised and the product is refluxed to obtain a product with a yield of more than 90% and a content of more than 98%.
Preparation [2,6-7]
Method 1: Solvent-free synthesis of phenyl chloroformate using molten phenol and phosgene as raw materials under the action of a small amount of catalyst:
![]()
Pour the melted phenol into a 500 mL four-necked flask equipped with a stirrer, thermometer, and spherical condenser tube, add the catalyst, start stirring, and start the circulating freezer. After the temperature rise is completed, keep it warm, introduce phosgene, and maintain the reaction temperature for 7 to 12 hours. Start taking a control sample at 7 hours of reaction, and analyze the area percentage of phenyl chloroformate and diphenyl carbonate through gas phase normalization. Take a medium control sample once every hour until the area percentage of phenyl chloroformate no longer increases and the reaction is over. Start cooling down and use nitrogen to drive out the residual phosgene from the system. After the phosgene is driven out, the material is distilled under reduced pressure. The positive component of phenyl chloroformate is collected when the vacuum degree is 0.096~0.098 MPa and the gas phase temperature is 102~104°C. The content is ≥98% through gas chromatography quantitative analysis.
Method 2: A two-step synthesis method for preparing pure phenyl chloroformate, which mainly includes the following steps:
1) Add 50g of molten phenol and 2.5g of the catalyst N,N-dimethylformamide into the reaction kettle, start stirring, and introduce 1.06 mol of phosgene at 100°C. After the reaction is complete, add activated carbon. Pour nitrogen into the reaction solution for about 20 minutes, and at the same time add an automatic ammonia spray system above the reaction kettle. If phosgene leaks, the ammonia spray system can automatically spray ammonia to minimize the hazard. After the reaction, the crude benzene chloroformate is obtained. Ester;
2) The obtained crude phenyl chloroformate is subjected to vacuum distillation to obtain pure phenyl chloroformate. The purity of phenyl chloroformate prepared by the above method is 95.6%, and the yield is 89.4%.
Method 3: Synthesis method of phenyl chloroformate using phenol as raw material. The production method includes the following steps:
1) Add 94Kg of molten phenol to the phenol dripping tank, first turn on the A and B cycle refrigerators connected to the reaction tank, lower the temperature of the lower part of the reaction tank to 0°C, and then add Sanguang to the lower part of the reaction tank. gas, and the amount of triphosgene added is 297-300Kg, add catalyst to the lower part of the reaction tank at the same time, the reaction time is 7 hours, take the middle control sample, and analyze the area percentage of phenyl chloroformate and the main by-product diphenyl carbonate through gas phase normalization;
2) Extract the above-mentioned medium control sample once every 1 hour, so that step 3 continues to react until the area percentage of phenyl chloroformate and the main by-product diphenyl carbonate no longer increases, and the number of extractions is 10 times;
3) After the above-extracted reaction tank is purged with nitrogen, the tail gas is passed into the phosgene decomposition tower to remove phosgene;
4) Distill out the phenyl chloroformate under reduced pressure from the degassed material.
Method 4: A production process for phenyl chloroformate, the production method includes the following steps:
1) Slowly increase the temperature of the stirring tank to 97-102°C, then introduce phosgene into the mixing tank from the middle of the side wall of the stirring tank, and the amount of phosgene introduced is 50-60 mol, and keep Continuously add phosgene, then open the phenol dripping tank, start stirring and open the catalyst spray tank at the same time, and add phenol at a rate of 70-140L/h, the reaction time is 2 hours, take a medium control sample, and analyze the gas phase normalization Area percentage of phenyl chloroformate and main by-product diphenyl carbonate;
2) Continue to introduce phosgene, drop phenol at a rate of 30-35L/h, and the reaction time is 4 hours. Take a medium control sample and analyze the gas phase normalization of phenyl chloroformate and the main by-product diphenyl carbonate. Area percentage of ester;
3) Continue to extract the above-mentioned medium control sample once every 30 minutes, so that the above-mentioned reaction continues until the area percentage of phenyl chloroformate and the main by-product diphenyl carbonate no longer increases, and the number of sampling times is 10-12 times;
p>
4) After the aforementioned final sampling is stopped, the stirring tank is degassed with nitrogen, and the tail gas is passed through the condenser to separate the organic solvent so that the content of the organic solvent is 5-8ppm, and then the tail gas is passed into phosgene for decomposition and absorption. Tower, that’s it;
5) Use the degassed material to distill the phenyl chloroformate under reduced pressure.
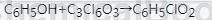
Main reference materials
[1] CN201610384240.5 A synthesis method of phenyl chloroformate using phenol as raw material
[2] Solvent-free synthesis of phenyl chloroformate
[3] CN201610402541.6 Preparation method of doxylamine impurity G
[4] CN201310440481.3 A new green process for synthesizing high-content nicosulfuron
[5] CN201210362552.8 Preparation method of tert-butyl carbazate
[6] CN201610319239.4 A two-step synthesis method for preparing pure phenyl chloroformate
[7] CN201610384248.1 A production process of phenyl chloroformate

 微信扫一扫打赏
微信扫一扫打赏

