Background and overview[1][2]
Organolithium compounds are an important class of organometallic compounds. They were prepared by K. Ziegler as early as 1929 by reacting organic halides with metallic lithium, and were subsequently used in organic synthesis. At present, organolithium compounds, as an important synthetic reagent, play an important role in basic theoretical research and industrial synthesis. Ziegler discovered that organolithium compounds have similar properties and application value to organomagnesium compounds (Grignard reagent) in organic synthesis, and in some aspects, compared with Grignard reagent, they have stronger reactivity, high yield, and smaller reduction tendency. , the product is easy to separate and can be dissolved in a variety of non-polar solvents, etc., so it can replace Grignard reagent in organic synthesis or make up for the deficiencies of Grignard reagent in certain syntheses. At the same time, organolithium compounds have unique properties in some organic synthesis, making them widely used and of great significance in organic synthesis.
Phenyllithium ignites spontaneously in air. Soluble in diethyl ether and appears as a dimer in solution. Phenyllithium is a commonly used organolithium compound. In the existing technology, it is generally produced by the reaction of bromobenzene or chlorobenzene and metallic lithium in ether or ether-benzene mixture, or by the reaction of diphenylmercury and metallic lithium in toluene. However, this method suffers from the fact that phenyllithium is too active and the raw materials are relatively more stable. When scaled up to industrial scale, the reaction is often difficult to initiate, the success rate is low, and the target product cannot be obtained. Due to the huge amount of phenyllithium used in basic research and industrial production and its irreplaceability, how to achieve large-scale production of phenyllithium has become an urgent problem that needs to be solved.
Apply[2-5]
Phenyllithium can be used as a metallizing reagent for the preparation of alkyl lithium, etc., and a dehydrohalogenation reagent for the preparation of vitamins and hormones, etc. Examples of its application are as follows:
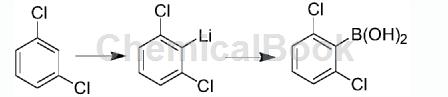
1. Preparation of 2,6-dichlorophenylboronic acid. This preparation method uses 1,3-dichlorophenyl ether as raw material. The raw material and lithiation reagent undergo lithium and hydrogen exchange reaction to prepare phenyllithium compound. The resulting benzene The phenylboronic acid ester is obtained by reacting the lithium compound with the boric acid ester; then the boric acid ester is hydrolyzed with dilute hydrochloric acid to obtain crude 2,6-dichlorophenylboronic acid; finally, pure 2,6-dichlorophenylboronic acid is obtained after purification product; this reaction uses tetrahydrofuran as the reaction solvent. The above-mentioned 2,6-dichlorophenylboronic acid preparation method has the advantages of using cheap and easily available raw materials, mild reaction conditions, simple post-processing operations, low production costs, high yields, reduced reaction costs, and is suitable for industrial production.
2. Preparation of 3-aldehyde-4-methylphenylboronic acid. This preparation method uses 2-methyl-5-bromobenzaldehyde as raw material, and reacts 2-methyl-5-bromobenzaldehyde with alcohol to obtain the acetyl boronic acid. Aldehydes and acetals undergo lithium halogen exchange reaction with lithiation reagents at low temperature to prepare phenyl lithium compounds. The resulting phenyl lithium compounds react with boronation reagents to prepare phenyl borate ester; then use hydrochloric acid with a mass concentration of 10% After hydrolysis, crude 3-aldehyde-4-methylphenylboronic acid is obtained, and finally purified to obtain pure 3-aldehyde-4-methylphenylboronic acid; tetrahydrofuran is used as the reaction solvent in this reaction. The above-mentioned 3-aldehyde-4-methylphenylboronic acid preparation method has the advantages of using cheap and easily available raw materials, mild reaction conditions, simple post-processing operations, low production costs, high yields, reduced reaction costs, and is suitable for industrial production.
3. Preparation of 2,3-difluorophenyl acetic acid, the specific steps are: (1) At low temperature, o-difluorobenzene reacts with organic lithium to generate o-difluorophenyllithium, and the o-difluorophenyllithium Then react with oxalate or chlorooxalate to generate 2,3-difluoroacetophenonate; (2) reduce the 2,3-difluoroacetophenolate under alkaline conditions, Then it is acidified and precipitated to obtain 2,3-difluorophenylacetic acid, which is then crystallized and purified. The above-mentioned method overcomes the shortcomings and defects of the existing synthesis technology. The process is simple, the reaction conditions are mild, the post-processing is simple and clean, the product purity is high, the cost is low, and it is suitable for industrial production.
4. Preparation of chlorbendarol and its salts, the preparation method includes the following steps: under an acidic catalyst, o-chloroacetophenone, paraformaldehyde, and dimethylamine hydrochloride are mixed in an organic solvent. Nisch reaction to prepare 1-o-chlorophenyl-3-dimethylamino-1-propanone hydrochloride; combine the obtained 1-o-chlorophenyl-3-dimethylamino-1-propanone hydrochloride with Neutralization reaction is carried out with alkali to obtain 1-o-chlorophenyl-3-dimethylamino-1-propanone; the obtained 1-o-chlorophenyl-3-dimethylamino-1-propanone is mixed with phenyl lithium in An addition reaction is carried out in an organic solvent to prepare chlorbendarol; and the prepared lorbendanol is reacted with hydrochloric acid to prepare chlorbendanol hydrochloride. The above preparation method is simple to operate, easy to control, has high safety performance, and can be used for industrial production.
Preparation [1]
A method for preparing phenyllithium, including the following steps:
1) Under the protection of inert gas, add sodium-lithium alloy and n-butyl ether to the system, and stir to obtain a sodium-lithium alloy dispersion; the mass fraction of sodium in the sodium-lithium alloy is 1 to 6% , the present invention uses sodium-lithium alloy to replace metallic lithium, which can promote the initiation of the reaction. The dosage mass ratio of sodium-lithium alloy and n-butyl ether is 1:10~30. The reaction process of sodium-lithium alloy and bromobenzene has relatively high requirements on moisture and air (such as oxygen, carbon dioxide). Before production, the air in the entire production system is replaced with an inert gas. The inert gas is argon. , the density of argon is higher than that of air, and the anhydrous and oxygen-free conditions are easy to control, the inert gas must be dried before use. The reaction between bromobenzene and sodium-lithium alloy is usually difficult to initiate, has a low success rate, and is very sensitive to temperature. The reaction stops when the temperature is below 10°C. Once the reaction stops, it cannot be saved even by raising the temperature and adding bromobenzene dropwise. At the same time, the temperature cannot be higher than 30°C, otherwise a large amount of coupling products will be produced.
(2) Add bromobenzene dropwise to the sodium-lithium alloy dispersion obtained in step (1) under stirring at 10-30°C, control the system temperature at 10-30°C, and after the dropwise addition is completed, the insulation reaction proceeds. Treat to obtain phenyllithium solution. Dissolve bromobenzene in n-butyl ether and then drip it into the system. The mass percentage concentration of the obtained bromobenzene n-butyl ether solution is 40 to 60%. In terms of active ingredients, the dosage of bromobenzene in the n-butyl ether solution is 10 to 20 times the mass of the sodium-lithium alloy in the system. The dropwise addition of bromobenzene is completed within 6 to 8 hours. The purity of the product is closely related to the dropping speed of bromobenzene. If the dropping speed is too fast, coupling products will be produced. During the dropping process of bromobenzene, the concentration of the product phenyllithium must be monitored in real time. When the product concentration does not increase, the dropwise addition of bromobenzene must be stopped. After the dropwise addition of benzene is completed, keep warm and continue the reaction for 1 to 2 hours.
Main reference materials
[1] A preparation method of phenyllithium
[2] CN200910129336.7 Preparation method of chlorbendarol and its hydrochloride
[3] CN201711471984.1 Preparation method of 3-aldehyde-5-methylbenzoic acid
[4] CN201810025201.5 Preparation method of 3-aldehyde-4-methylphenylboronic acid
[5] CN201711442610.7 A preparation method of 2,6-dichlorophenylboronic acid

 微信扫一扫打赏
微信扫一扫打赏

