Background and overview[1][2]
Benzoylformic acid is a colorless or light yellow crystal that decomposes by distillation under normal pressure and is soluble in water, acid, ether and hot carbon tetrachloride; it can be used to synthesize a variety of medicines and pesticides. After oxidation and ammoniation, benzoylformic acid can be used to prepare α-amino acids, which can be used to synthesize physiologically active pharmaceutical peptides to prevent and treat hypertension and cardiovascular diseases. It reacts with ammonia and hydrazine derivatives to prepare aryl triazoline and triazine compounds, which are used as herbicides. It can be used as a sensitizer for fluorescent materials and a catalyst for organic oxidation reactions by complexing with metal elements. In addition, benzoylformic acid is also a component of the synthesis of certain important hormones in the organism. These hormones can regulate the oxidation process of sugar in the body and maintain the balance of fat and sugar in the body. In recent years, through complexing with metal elements, it has been found that it can be used as a sensitizer for fluorescent materials and a catalyst for organic oxidation reactions. It has attracted people’s attention and is a promising organic synthesis intermediate.
Apply[3]
1. In terms of preparing pharmaceutical intermediates: using benzoylformic acid or its esters as raw materials, dozens of chemical raw materials can be directly synthesized. Benzoylformate is an intermediate in the drug orphenium bromide for the treatment of gastric and duodenal ulcers. There are generally two types of production processes for these drugs. One is to prepare benzoylformic acid by first forming an ester with the corresponding alcohol, and then reacting with Grignard reagent. For example, weichangin is used for gastric acid and gastric ulcers, cyclomandelate is used as an antithrombotic drug to treat cerebrovascular disease, urodolin is an antispasmodic drug for treating polyuria and frequent urination, and atropine is an anticholinergic drug. The other type is where benzoylformic acid first reacts with the corresponding amine to form an amide, and then is cyclized to prepare it, such as the central nervous system stimulant pemoline and the antidepressant mirtazapine.
Another important use of benzoylformic acid in medicine is the preparation of chiral mandelic acid. Chemically synthesized almonds are all racemates, and chemical separation or chromatographic separation is difficult and expensive. Using biotechnology with mild conditions, R-(-)-mandelic acid is synthesized in one step starting from benzoylformic acid. The content of R-(-)-mandelic acid in the total mandelic acid is higher than 98.0%.
2. As a pesticide intermediate, benzoylformic acid is mainly used to synthesize triazone herbicides. For example, benzotrione and phenazine are used.
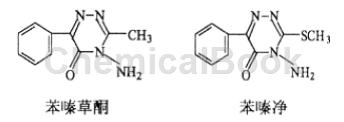
Mediatrione is a dryland herbicide developed by a German company. It is mainly suitable for weeding in sugar beet fields. It is an efficient and low-toxic pesticide and has a broad market in Europe and Northeast my country. Difenzin is an analogue of dipyramide, used for soil, stem and leaf treatment, and control of broadleaf weeds. The synthesis of benzodiazepine starts from benzoylformic acid and forms methyl benzoylformate with methanol, and then condenses with acetyl hydrazine to form hydrazone. It is chlorinated with phosphorus pentachloride and finally cyclized with hydrazine hydrate. The synthesis of benzoylformate also starts from benzoylformic acid, which is first prepared with methanol to form methyl benzoylformate, and then directly cyclized with thiosemicarbazone. Because benzoylformic acid contains a carbonyl group, it can complex with metal ions to form a complex. In a mixed rare earth complex with benzoylformic acid and o-phenanthroline as ligands, terbium Tb3+, europium Eu3+ and inert ions (La3+, Y3+ and Gd3+) as central ions, the non-luminescent ion La3+ contributes to the fluorescence emission intensity of the complex It has a great influence and can significantly enhance its relative fluorescence emission intensity, which provides a theoretical basis for people to search for luminescent materials with good luminescence performance and low price.
3. Uses in other fields. Because benzoylformic acid contains a carbonyl group, it can complex with metal ions to form a complex. In a mixed rare earth complex with benzoylformic acid and o-phenanthroline as ligands, terbium Tb3+, europium Eu3+ and inert ions (La3+, Y3+ and Gd3+) as central ions, the non-luminescent ion La3+ contributes to the fluorescence emission of the complex. The intensity has a great influence, which can significantly enhance the relative fluorescence emission intensity, which provides a theoretical basis for people to search for luminescent materials with good luminescence performance and low price. Benzoylformic acid reacts with the divalent iron salt of the derivative of a heterocyclic compound to form a ferroprotoporinase complex, which is used as a catalyst for the oxidation reaction. Ultraviolet light-cured powder coating (referred to as UV-cured powder coating) is a new technology that combines traditional powder coating and UV curing technology. It is especially suitable for low-temperature coatings that require environmental protection.�In situations of rapid curing and high-efficiency production. The photoinitiator in UV-curable powder coatings synthesized from benzoylformate is suitable for preparing thicker cured materials and can obtain coatings with good decorative effects and excellent weather resistance.
Preparation [3]
Benzoylformic acid is an a-keto acid and is easily oxidized, decarbonylated and decarboxylated, so its synthesis is difficult. Early preparation methods were all laboratory methods, such as biological fermentation oxidation of mandelic acid and phenylacetylene selenium dioxide oxidation, which were extremely costly; the acetyl palladium-catalyzed iodobenzene dicarbonylation reaction had harsh conditions and could not be industrialized. At present, the main synthesis methods that can be industrialized are:
1) Benzoylnitrile hydrolysis method
Benzoylnitrile is hydrolyzed under the catalysis of concentrated hydrochloric acid to form benzoylformic acid. The reaction is as follows:
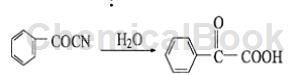
Process flow: Put benzoyl nitrile, sodium chloride and 85% sulfuric acid into the reaction kettle according to the amount of substances 1:0.1:1.2, stir and dissolve, then raise the temperature to 50℃ and react for 10 hours; then Cool to room temperature and extract three times with ethyl acetate. Combine the extracts, wash with 5% sodium bicarbonate solution, heat the oil layer to remove the solvent, and cool to obtain a crude product. Dissolve the crude product in hot carbon tetrachloride and use activated carbon to decolorize it; filter out the activated carbon particles, cool the filtrate to normal temperature first, then use frozen brine to lower it to 0℃, and filter to obtain the finished product . The content is more than 97%, and the yield is 80%. This method is simple to operate, has mild conditions and has a high yield. It is a method commonly used abroad. Domestic raw material benzoyl nitrile is difficult to source and expensive, so the output of this process is very small.
Main reference materials
[1] Research progress on the synthesis technology of benzoylformic acid and its esters
[2] CN201510639934.4 Synthesis method of benzoylformic acid
[3] Synthesis and application of benzoylformic acid

 微信扫一扫打赏
微信扫一扫打赏

