Background and Overview
2-Amino-3-fluorophenol is a phenol derivative and can be used as a pharmaceutical intermediate.
Preparation method[1-2]
Method 1,
Step 1 1-fluoro-3-methoxy-2-nitrobenzene:
To a solution of 1,3-difluoro-2-nitrobenzene (100g, 0.63mol) in MeOH (1.3L) at 0°C, slowly add MeONa solution (0.69mol, in MeOH, composed of 15.9g metallic sodium and 200 mL MeOH freshly prepared). The resulting reaction was stirred at room temperature for approximately 15 hours, then the reaction mixture was concentrated and diluted with EtOAc. The organic phase was washed with water and brine successively, dried with Na2SO4, then filtered and concentrated in vacuo to obtain 1-fluoro-3-methoxy-2-nitro Benzene (it was used without further purification. Yield 98g, 91.4%.1H -NMR (CDCl3, 400MHz) δ7.38 -7.44 (m, 1H), 6.72-6.88 (m, 2H), 3.95 (s, 3H).
Step 2 3-Fluoro-2-nitrophenol:
Add BBr3 solution dropwise to a solution of 1-fluoro-3-methoxy-2-nitrobenzene (98g, 0.57mol) in dichloromethane (500mL) at -40°C. (Stir the resulting reaction at room temperature for about 15 hours, then slowly pour the reaction mixture into ice water (500mL), and extract the resulting solution with EtOAc (300mL×3). The combined organic layers are sequentially washed with 5% NaHCO3< Washed with aqueous solution and brine, then dried over Na2SO4, filtered and concentrated in vacuo to give 3-fluoro-2-nitrophenol, which was treated without further It can be used after purification. Yield 85g, 95%, 1H-NMR (CDCl3, 400MHz) δ7.43-7.49 (m, 1H), 6.88 (d, J = 8.0Hz, 1H), 6.73-6.78 (m, 1H).
Step 3 2-amino-3-fluorophenol:
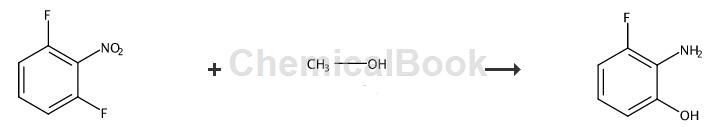
3-Fluoro-2-nitrophenol (38g, 0.24mol) was dissolved in EtOH, then palladium on carbon (5g, 10% Pd) was added. The reaction flask was evacuated and the reaction mixture was placed under H2 atmosphere (1 atm) and stirred at room temperature for 3 hours. The reaction mixture was then filtered through a short pad of Celite and the Celite was washed with EtOH. The combined filtrate and washes were concentrated in vacuo to give 2-amino-3-fluorophenol, which was used without further purification. Yield 26g, 85.7%, 1H-NMR (DMSO, 400MHz) δ9.43 (s, 1H), 6.42-6.53 (m, 2H), 6.32-6.42 (m, 1H), 4.34 (s, 2H).
Method 2,

Add stannous chloride dihydrate (0.724g, 3.18mmol) to a solution of 3-fluoro-2-nitrophenol (0.100g, 0.636mmol) in THF (5.0mL) and water (5.0mL). The mixture was heated to 80°C for 40 minutes. After cooling to room temperature, the reaction mixture was diluted with ethyl acetate and saturated sodium bicarbonate solution. The mixture was then filtered to remove insoluble material and the layers were separated. Extract the aqueous layer three times with ethyl acetate. The extract was washed with brine, dried over sodium sulfate, decanted and concentrated to give the product which was used without further purification (65 mg, 80%). 2-Amino-3-fluorophenol, yield 65 mg, 80%, LCMS (M+H): 128.0.
Main reference materials
[1] PCT Int. Appl., 2011106992, 09 Sep 2011
[2] PCT Int. Appl., 2010135650, 25 Nov 2010

 微信扫一扫打赏
微信扫一扫打赏

