Background and overview[1]
Methyl 3,4-dimethoxy-5-hydroxybenzoate is an ester derivative and an important intermediate in the synthesis of acotiamide. Acotiamide hydrochloride, chemical name is N-{2-bis(1-methylethyl)aminoethyl}-2-(2-hydroxy-4,5-dimethoxybenzoyl) ) Aminothiazole-4-carboxamide hydrochloride, which is a new type of M1 and M2 receptor antagonist developed by Japan’s Zeria New Pharmaceutical Co., Ltd., was launched in Japan on June 6, 2013 by Astellas Pharmaceuticals and Zeria Pharmaceuticals Jointly launched and approved for marketing, it became the world’s first functional dyspepsia (FD) treatment drug. This product is approved for the treatment of postprandial fullness, epigastric distension and early satiety caused by FD. As the world’s first drug to treat FD, this drug has naturally attracted widespread attention.
Preparation[1]
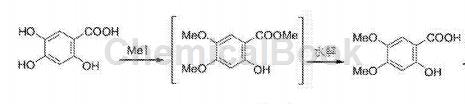
Add 2,4,5-trihydroxybenzoic acid (51.1g, 0.3mol), sodium hydroxide (40g) and 1000mL of ethanol into the reaction bottle, stir for half an hour, and slowly add 62.3mL of iodomethane ( 1mol), after the dropwise addition is completed, heat to 50°C and stir for 5 hours. TLC monitors the reaction to be completed. The reaction solution is naturally cooled, the solvent is evaporated under reduced pressure, 2000mL of water is added, extracted with 3×1500mL of ethyl acetate, and the organic layers are combined. Wash with 3×2000mL water until the water layer is colorless, dry with anhydrous MgSO4, filter, and concentrate under reduced pressure to obtain 60.7g of 2-hydroxy-4,5-dimethoxybenzoic acid methyl Ester, HPLC purity was 99.4%, yield 94.8%.
Apply [2]
3,4-Dimethoxy-5-hydroxybenzoic acid methyl ester can be used to prepare acotiamide hydrochloride, the route is as follows:
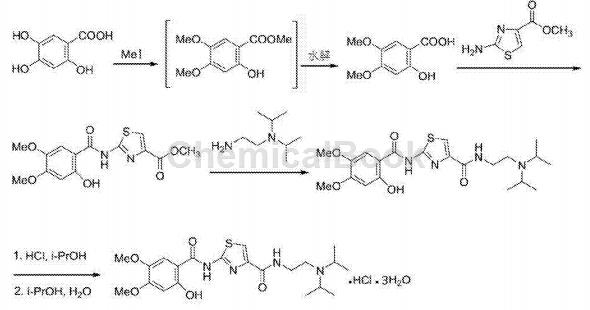
Step 1. Synthesis of 2-[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-4-thiazolecarboxylic acid methyl ester
39.8g 2-hydroxy-4,5-dimethoxybenzoic acid (0.2mol), 34.8g 2-aminothiazole-4-carboxylic acid methyl ester (0.22mol) and 42.1g CDMT (0.24mol) were suspended in a In the reaction bottle of 800mL N,N-dimethylformamide, add dropwise a mixed solution composed of 15.8g pyridine (0.2mol) and 200mLN,N-dimethylformamide under stirring in an ice-water bath. Complete the addition within 20 minutes and then continue. Stir for 1 hour, then warm to room temperature and stir for 2 hours. Stop stirring, add the reaction solution to water and beat, crystallize in an ice bath, filter, and dry under reduced pressure to obtain a white solid 2-[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-4- Thiazole carboxylic acid methyl ester 64.5g, yield 95.2%, HPLC purity 99.8%.
Synthesis of acotiamide hydrochloride trihydrate
Place 50.9g (0.15mol) of 2-[(2-hydroxy-4,5-dimethoxybenzoyl)amino]-4-thiazolecarboxylic acid methyl ester and 1000ml DMF in a four-necked bottle, N Under air protection, add 21.6g N,N-diisopropylethylenediamine (0.15mol), heat to 100°C and react for 5 hours. Stop the reaction and evaporate the solvent to dryness under reduced pressure. 300 mL of n-butanol was added to the residue, and then washed with 10% sodium bicarbonate and saturated brine in sequence. After washing, the organic phase was retained. 150 mL of isopropyl alcohol was added to the obtained organic phase. Then a salt-forming reaction is carried out, and the salt-forming reaction is carried out under ice-water bath conditions. Use concentrated hydrochloric acid to adjust the pH of the system to 1 to 2. After stirring for 4 hours, white solid came out of the systemPrecipitate. The solid was collected by suction filtration and dried to obtain 69.4g of white powdery solid acotiamide hydrochloride trihydrate, with a molar yield of 85.3%, an HPLC purity of 99.8%, and a total impurity of less than 0.1%.
Main reference materials
[1] CN201610696259.3 A preparation method of acotiamide hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

