Background and overview[1][2]
With the advancement of fluorine chemical synthesis technology and in-depth research on its mechanism of action, it has been discovered that fluorine-containing pesticides have the advantages of low dosage, strong selectivity, low residue, easy degradation, and environmental friendliness, making it a popular choice at home and abroad. One of the hot spots in the field of new agricultural development. At present, fluorine-containing pesticides account for more than 20% of the more than 1,300 pesticide technical materials in the world. Among fluorine-containing pesticides, herbicides account for about 45%, insecticides account for 33%, fungicides account for about 15%, and others account for 7%. Fluorine-containing herbicides are the most developed in recent years, and have been developed in the past 10 years. Fluorinated compounds account for 50% of herbicides. As for the development direction of herbicides as pesticides, it can be seen that fluorinated pesticides have become the focus of the development of the world’s pesticide industry. The in-depth development of fluorine-containing pesticides has greatly promoted the development and application of fluorine-containing pesticide intermediates, resulting in the rapid development of the fluorine-containing pesticide and intermediates industry.
2-Chloro-4-fluoroacetanilide is a key intermediate of fluorine-containing pesticides. It can be used to synthesize high-efficiency, non-toxic, and low-residue new fluorine-containing pesticides. This type of new pesticides can be widely used in rice, The prevention and control of crop pests such as vegetables, fruits, sugar cane, and corn, as well as agricultural pests such as cockroaches and termites, can effectively prevent pests that are resistant to pesticides. It is the best alternative to highly toxic pesticides such as methamidophos and is relatively safe for humans and animals. When multiple pests occur at the same time, multiple treatments with medicine can be achieved to achieve better control effects.
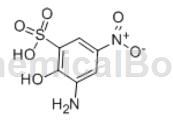
2-Chloro-4-fluoroacetanilide
Preparation[2]
After searching, there are currently few preparation methods for 2-chloro-4-fluoroacetanilide reported in patent and non-patent literature at home and abroad. The main preparation methods are using p-fluoroaniline as raw material through chlorination and acetylation. And a preparation method using 2-chloroacetanilide as raw material through fluorination. The preparation methods currently reported in the literature are mainly as follows:
1. Products obtained by acetylation and chlorination of p-fluoroaniline as raw materials
This process uses p-fluoroacetaniline as the starting material. After acetic anhydride or acetyl chloride is acetylated to p-fluoroacetanilide, it is then chlorinated with Br2, reduced with sodium sulfite or other reducing agents, and then filtered, washed, and reconstituted. After crystallization, the target product 2-chloro-4-fluoroacetanilide (BFA) can be obtained. This process scheme has the advantages of readily available raw materials, scientific process, simple operation, low cost, and can obtain high yield and high purity 2-chloro-4-fluoroacetanilide (BFA).
2. Use p-fluoroaniline as raw material to obtain products through chlorination and acetylation
This method is a synthesis method reported in European patents. 2-chloro-4-fluoroaniline (BFA) is obtained by direct chlorination of p-fluoroaniline, and then acylated with acetyl chloride to obtain 2-chloro-4-fluoroacetyl Aniline (BFA), yield 49%. The disadvantage of this method is that it first needs to obtain relatively high content of 2-chloro-4-fluoroaniline (BFA). When using direct chlorination of p-fluoroaniline, its selectivity is relatively low. Poor, due to the presence of amino groups, the main product generated during direct chlorination is 2,6-dichloro-4-fluoroaniline (BFA). This process method does not meet the requirements of industrial production.
3. Products obtained by chlorination using p-fluoroacetanilide as raw material
This method was first developed by Michael J.S in 1962. It uses p-fluoroacetanilide as the raw material and glacial acetic acid as the reaction solvent. The acetic acid solution of Br2 is added dropwise to the reaction system, reacted at 60°C for 1 hour, and filtered Recrystallized from ethanol aqueous solution, the yield was 94%. This method has the advantages of simple process and high yield, but because the HBr generated during the reaction cannot continue to participate in the reaction, the chlorine atom economy is poor and the wastewater treatment pressure is high.
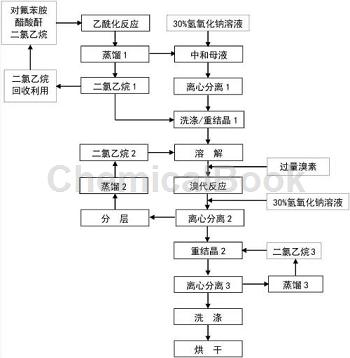
Method 1:
Step 1. In the reaction kettle 1, first vacuum feed and add dichloroethane as the solvent;
Step 2: Add a certain amount of p-fluoroaniline through metering tank A. After the addition is completed, turn on the stirring device so that p-fluoroaniline is evenly mixed in dichloroethane;
Step 3: Feed a certain amount of acetic anhydride into metering tank B, turn on the dripping device, and add acetic anhydride dropwise into reactor 1. The reaction will generate heat;
Step 4: When the acetylation reaction between acetic anhydride and p-fluoroaniline is complete, pour cooling water into the jacket of the reactor, stir and cool down within half an hour;
Step 5: Feed the measured Br2 into metering tank C, turn on the dripping device, and add Br2 to reactor 1.�Add Br2, the chlorination reaction begins, and keep the temperature at 25-30°C;
Step 6: After all the dropwise addition of Br2 is completed, transfer the materials in reactor 1 to reactor 2, repeat steps 1 to 5 in reactor 1, and proceed with subsequent reactions in reactor 2;
Step 7: Feed a certain amount of hydrogen peroxide into the metering tank D connected to the reactor 2, and add it dropwise into the reactor 2 while turning on the stirring. After the dripping is complete, stir for 3 hours;
Step 8: Send a quantitative amount of the pre-prepared sodium sulfite solution into the metering tank E connected to the reactor 2 to reduce the oxidative substances in the synthetic liquid after the oxidative chlorination reaction in the reactor 2;
Step 9: Transfer the reaction product and synthesis mother liquor in reactor 2 to a centrifuge, perform centrifugal filtration, and then wash the filter cake with a certain amount of dichloroethane;
Step 10: Add the washed filter cake into the reaction kettle, add a certain amount of dichloroethane for recrystallization and purification, and then centrifuge to obtain high-purity 2-chloro-4-fluoroacetanilide.
Method 2:
In a 2000L reaction kettle, put 355kg (3.2kmol) of p-fluoroaniline, stir and dropwise add 333kg (3.3kmol) of acetic anhydride. When the reaction exotherm rises to 105~110℃, control the dropping speed. Within this temperature range Finish dripping within 1.5 hours and keep warm for 1 hour. Sampling is controlled, the raw material p-fluoroaniline is less than 0.5%, the acylation reaction is completed, and the acetic acid is slowly steamed out under reduced pressure (no steaming out until -0.09Mpa). By-product acetic acid with a content greater than 97wt% is obtained.
Put 800kg of chlorobenzene into the above reaction kettle while it is hot, then cool it to 40~45°C and add 648kg (3.8kmol) of hydrochloric acid (concentration 48wt%). After stirring for half an hour, cool down to 35~40°C and start dripping. Add 544kg (4.8kmol) of hydrogen peroxide (concentration: 30wt%). The reaction will be exothermic. Control the dropping speed to keep the reaction temperature at 40-45°C. After the dripping is completed, keep it at this temperature for 3 hours. Sampling should be controlled to ensure that the intermediate acylate is less than 0.5%. Otherwise, add hydrogen peroxide until it is qualified. At this time, 2,6-dichloro-4-fluoroacetanilide is less than 0.1%.
Cool the temperature, add dropwise 160kg aqueous solution containing 40kg sodium sulfite below 35℃, freeze and cool to -5~5℃, suction filter, rinse with 160kg ice chlorobenzene to obtain the crude product.
After adding the crude product to a methanol aqueous solution with a concentration of 80wt% to dissolve it, adjust the pH to 7-8 with a sodium hydroxide aqueous solution with a concentration of 30wt%, cool to 0℃, crystallize and filter, wash with a small amount of solvent, and filter the cake at 60℃ After drying, 632kg of white crystalline finished product was obtained, with a yield of 85.2% and a purity of 99.8% (HPLC).
Main reference materials
[1] Yin Lihua, & Yao Lulu. (2015). A review of research on the synthesis process of 2-bromo-4-fluoroacetanilide. Chemical Management (6), 171-172.
[2]Li Xiaoxu. (2009). Research on the synthesis of several important fluorine-containing intermediates. (Doctoral dissertation, Tianjin University).
[3]Wu Ruibin, & Lu Tao. (2007). Synthesis study of romacoxib. Progress in Pharmaceutical Sciences, 31(12), 573-575.

 微信扫一扫打赏
微信扫一扫打赏

