Background and Overview
3-Fluoro-4-methylanisole is an ether organic compound that can be used in organic synthesis.
Preparation[1]

A mixture of 5-methoxy-2-methylaniline (5.0 g; 36 mmol), HCl (7.6 mL, 12 M solution; 91 mmol) and H2O (11 mL) was stirred at 60 Heat at ℃ for 15 minutes until dissolved. Cool the reaction to 0°C and add aqueous NaNO2 solution (2.5g; 36mmol) dropwise (internal temperature = 7°C). The reaction was stirred at 0 °C for 30 min and a solution of HBF4 at 0 °C (5.3 mL of a 48% solution; 40 mmol) was carefully added. The reaction was stirred at 0°C for 20 minutes, and the resulting brown solid was filtered and washed with ice water (3 × 10 mL) and H2O (2 × 10 mL). The solid was dried under high vacuum for 20 hours and then heated (heat gun) until the release of BF3 (white smoke) ceased. The resulting brown oil was partitioned between EtOAc and H2O. The organic phase was dried (Na2SO4), concentrated in vacuo and distilled by Kugelrohr to give 3-fluoro-4-methylanisole (1.6g; 31%) , as colorless oily substance.
Apply [2]
Salvi et al. reported that it can be used to prepare a diarylphosphine ligand L10b. The reaction formula is as follows:
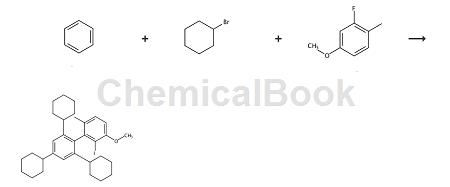
Step 1
In a 500mL three-neck round bottom flask, add aluminum trichloride (38g, 285mmol) and benzene (8mL, 89mmol) in CH2Cl2 (60mL ) and cool the suspension to 0°C. Cyclohexyl bromide (35 mL, 285 mmol) was added dropwise. Stir the reaction for 2 hours, slowly warming to room temperature (if the reaction is stirred at room temperature for longer, the yield of product decreases sharply). The reaction mixture was then carefully quenched with 50 mL of ice and diluted with 200 mL of diethyl ether. The layers were separated and the ether layer was washed twice with 50 mL of water and 50 mL of brine. The organic layer was then dried over MgSO 4, filtered and concentrated. The resulting colorless oily mixture was filtered through a plug of silica to obtain 1,3,5-tricyclohexylbenzene with a purity of 80% by GC and used as such in the next step. Yield 24g, 84%. 1H NMR (400MHz, CDCl3) δ: 6.87 (s, 3H), 2.53-2.38 (m, 3H), 1.92- 1.77 (m, 15H), 1.72 (d, J = 12.4Hz, 4H), 1.48-1.29 (m, 14H), 1.29-1.17 (m, 4H) ppm – the integral is slightly off due to impurities. 13 C NMR (101MHz, CDCl3) δ: 148.0, 123.13, 45.0, 34.5, 27.2, 26.5ppm.
Step 2
The crude mixture containing 1,3,5-tricyclohexylbenzene (19.5g, 60mmol) was dissolved in 20mL CHCl3 under argon and cooled to 0°C, then heated at 15 Bromine (3 mL, 60 mmol) was slowly added at ℃. minute. Warm the solution to room temperature and stir until complete (usually less than 2 hours as determined by GC). The reaction mixture was diluted with 50 mL of dichloromethane, and the resulting solution was washed with water. Extract the aqueous layer with dichloromethane (twice), combine the organic layers, wash with 50 mL 10% NaOH and brine successively, then dry with MgSO4, filter, and concentrate to obtain a yellow solid. Then set the firmwareTriturate with ethyl acetate and collect the resulting white solid by filtration and dry under vacuum. 2,4,6-Tricyclohexylbromobenzene (L8a), yield 14g, 58%. Melting point 160-165℃. 1H NMR (400MHz, CDCl3) δ: 6.95 (s, 2H), 3.08 (tt, J = 11.5, 3.0Hz, 2H), 2.48-2.45 (s, 1H), 1.98-1.69 (m, 15H), 1.55 – 1.17 (m, 15H) ppm. 13 C NMR (101MHz, CDCl3) δ: 147.1,146.5,124.2,123.6,44.7,44.3,34.7,33.8,27.2, 26.6,26.4 ppm.
Step 3
Synthesis of 2-iodo-2′,4′,6′-tricyclohexyl-3-methoxy-6-methylbiphenyl (L10b):
An oven-dried 500mL three-neck round-bottom flask equipped with a magnetic stirring rod was filled with magnesium chips (1.17g, 48.0mmol) and 2,4,6-tricyclohexylbromobenzene (16.0g, 40.0mmol). And equipped with reflux condenser, glass stopper and rubber diaphragm. Purge the flask with argon and add THF (60 mL) via syringe. The reaction mixture was heated to reflux and 1,2-dibromomethane (40 μL) was carefully added via syringe. The reaction mixture was stirred at reflux for 1.5 hours and then allowed to cool to room temperature. A separate oven-dried 2L round-bottom flask equipped with a magnetic stir bar and equipped with a rubber septum was purged with argon, followed by THF (200 mL) and 2-fluoro-4-methoxy-1-methylbenzene (2.8 g , 20.0 mmol) synthesized according to the reported protocol was added via syringe.
Cool the reaction mixture to -78 °C and add n-BuLi (2.5 M in hexanes, 8.1 mL, 20.2 mmol) dropwise over 40 min. The solution was stirred at -78°C for 1 hour, then the Grignard reagent prepared in the first reaction vessel was added via cannula within 30 minutes, and the reaction mixture was stirred at -78°C for 1 hour (containing Grignard reagent Containers must be maintained at 45°C while cannulated to avoid reagent precipitation). The reaction mixture was then slowly warmed to room temperature and stirred for a further 12 hours. The reaction mixture was then cooled to 0 °C and a solution of iodine in THF (1 M, 40 mL, 40.0 mmol) was added via syringe over 15 min, the resulting dark red solution was warmed to room temperature and stirred for 1 h. The solvent was removed by rotary evaporator, A yellow solid was obtained, which was dissolved in CH2Cl2 and washed successively with saturated sodium sulfite solution (freshly prepared) and brine. The organic layer was then dried with MgSO4, filtered, and the solvent was removed by rotary evaporator to obtain a yellow solid. The crude material was dry loaded onto silica gel and purified by flash chromatography (silica gel, 0-20% ethyl acetate in hexane).
In addition to the desired product, 2,4,6-tricyclohexyliodobenzene can also be recovered and reused. Obtain the desired product. Yield 9.2g, 80%, white crystalline solid. Melting point 200-202°C. 1H NMR (400MHz, CDCl3) δ: 7.18 (d, J = 8.3Hz, 1H), 7.02 (s, 2H) , 6.74 (d, J = 8.3Hz, 1H), 3.93 (s, 3H), 2.59- 2.49 (m, 1H), 1.99 (s, 2H), 1.95 (s, 3H), 1.94-1.90 (m, 2H ), 1.89-1.85 (m, 3H), 1.81-1.73 (m, 1H), 1.72- 1.54 (m, 9H),
Main reference materials
[1] PCT Int. Appl., 2001021602, 29 Mar 2001
[2] From Organic Letters, 14(1), 170-173; 2012

 微信扫一扫打赏
微信扫一扫打赏

