Background and Overview
3-Chloro-2-methoxybenzoic acid is a benzoic acid compound that can be used in organic synthesis.
Preparation[1]
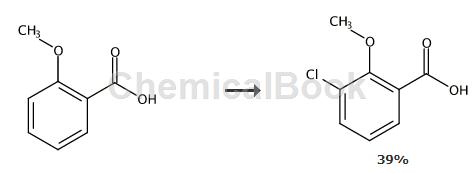
General method for preparing 3-substituted 2-methoxybenzoic acid (5a-g): A solution of o-anisic acid (0.46g, 3mmol) in THF (5mL) was added dropwise to a stirred n-78°C A solution of BuLi/t-BuOK (1:1, 12mmol) in THF (30mL). After stirring at -78 °C for 2 h, the reaction mixture was quenched with a solution of electrophile (18 mmol) in THF (5 mL). Stirring was maintained for 1 hour and the resulting mixture was warmed to room temperature and hydrolyzed with water (30 mL). The aqueous layer was washed with diethyl ether (2 × 30 mL), acidified with (2M) HCl aqueous solution until the pH reached 1, and extracted with diethyl ether (3 × 30 mL).
The organic layer was dried with MgSO4, filtered, and concentrated in vacuo to obtain crude benzoic acid, which was purified by chromatography or fractional crystallization. Preparation of 3-chloro-2-methoxybenzoic acid According to the above general procedure, the THF solution of recrystallized o-anisic acid (1,0.46g, 3mmol) was added dropwise to the stirring -78℃n-BuLi/t-BuOK (1:1,12mmol) solution. The solution was quenched with hexachloroethane (4.26 g, 18 mmol). Work-up in the usual way followed by chromatography (cyclohexane/ethyl acetate 80:20) gave 3-chloro-2-methoxybenzoic acid (0.218 g, 39%) as a yellow solid: mp 114- 116℃. 1H NMR (200MHz, CDCl3) δppm: 4.06 (s, 3H), 7.21 (t, 1H, J = 8.0Hz), 7.62 (dd, 1H, J = 8.0Hz, J = 1.6Hz), 7.99 (dd), 1H, J = 8.0Hz, J = 1.6Hz). 13CNMR (100MHz, CDCl3) δppm: 62.5, 124.6, 125.3, 129.0, 131.2, 135.7, 155.9, 167.6. IR (pure): 2826,2558,1667,1586,1463,1234,1076,917cm-1. HRMS (EI) m/z calculated value. C8H7O3Cl (M + .bul): 186.0083. Actual measurement: 186.0079.
Apply [2]
WO2011140425 reported that 3-chloro-2-methoxybenzoic acid can be used to prepare heterocyclic fused spiro[chromene-piperidine] derivative ion channel modulators. Examples of preparation methods are as follows:
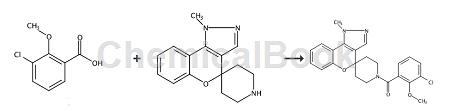
9-Aza-1-methyl-1H-spiro[benzo[4,3-c]pyrazole-4,4′-piperidine] (64 mg, 0.19 mmol), 3-chloro-2 – A solution of methoxybenzoic acid (45 mg, 0.23 mmol), HATU (89 mg, 0.23 mmol) and DIPEA (169 μL, 0.97 mmol) in DMF (0.5 mL) was stirred at room temperature for 16 h. Purification by HPLC (10-90% MeOH in water (HCl modifier)) afforded the HCl salt of the product. Dissolve the material in EtOAc, wash with saturated aqueous Na2CO3 (2x), brine, and wash with Na2SO 4 Dry and evaporate to dryness to obtain (3-chloro-2-methoxyphenyl) (1-methylspiro[[1]benzopyrano[4,3 C]pyrazole-4 (1H),4′-piperidin]-1′-yl)methanone.
Main reference materials
[1] Journal of Organic Chemistry, 72(9), 3419-3429; 2007
[2] PCT Int. Appl., 2011140425, 10 Nov 2011

 微信扫一扫打赏
微信扫一扫打赏

