Background and overview[1]
4-(6-(Acryloyloxy)hexyloxy)benzoic acid can be used as a pharmaceutical synthesis intermediate. If 4-(6-(acryloyloxy)hexyloxy)benzoic acid is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and wash skin thoroughly with soap and water, e.g. If you feel unwell, seek medical attention; if contact with eyes occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
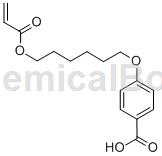
Preparation method[1]
The preparation of 4-(6-(acryloyloxy)hexyloxy)benzoic acid is as follows:
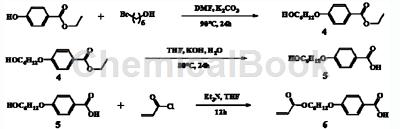
The specific synthesis steps are as follows:
(1) Preparation of intermediate 4
Weigh 9.9-10.0g ethyl parahydroxybenzoate, add 80mLDMF to dissolve it, add 9.9-10.2g potassium carbonate, heat and stir for half an hour, add 10.8-10.9g 6-bromohexanol, and control the reaction temperature90-92℃, reflux reaction for 24 hours, cool to room temperature after the reaction is completed, pour the system into 600mL water to dilute, extract with ether, dry, and rotary evaporate to obtain intermediate 4;
(2) Preparation of intermediate 5
Weigh 12.0-12.2g of intermediate 4 prepared in step (1), add 100mL of THF to dissolve it, then add 4.8-4.9g of NaOH aqueous solution and 50mL of it, and control the reaction temperature to 85-90°C. Reflux the reaction for 24 hours. After the reaction is completed, cool to room temperature, filter with suction, add to dissolve, adjust the pH to precipitate the product, filter with suction, and dry under vacuum to obtain intermediate 5;
Preparation of (3)4-(6-(acryloyloxy)hexyloxy)benzoic acid
Weigh 5.0-5.1g of intermediate 5 prepared in step (2), add 500mLTHF to dissolve it, add 7.1-7.2mL triethylamine, and add 1.5mL acryloyl chloride dropwise in an ice bath,0 ℃Stir for 30 minutes and then return to room temperature and stir overnight. After the reaction is completed, the system is concentrated, diluted with water, and 4-(6-(acryloyloxy)hexyloxy)benzoic acid is obtained through extraction and silica gel column chromatography.
Main reference materials
[1] CN201710596291.9 A highly polarized fluorescent film and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

