Background and Overview
2-Bromo-5-hydroxymethylphenol is an organic synthesis intermediate and pharmaceutical intermediate that can be used in laboratory research and development processes and chemical and pharmaceutical synthesis processes.
Preparation[1]
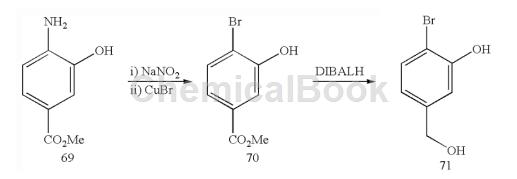
Methyl 4-bromo-3-hydroxybenzoate (70): To a suspension of methyl 4-amino-3-hydroxybenzoate 69 (21.7 g, 127 mmol) in water (115 mL) at 0°C Add a solution of sodium nitrite (9.1 g, 127 mmol) in water (50 mL). The resulting mixture was stirred at 0°C for 1 hour. Meanwhile, dissolve copper sulfate pentahydrate (42.4g, 169mmol) in water (135mL) under heating and slowly add sodium bromide (26.4g, 257mmol) under stirring.
The resulting dark green solution was stirred for 5 minutes and then treated with a solution of sodium sulfite (11.3 g, 90 mmol) in water (40 mL). The resulting lime green slurry was stirred for 5 minutes and then cooled by adding ice. Decant off the ice water, being careful to avoid drying it out. The residue was washed with water (3x) and the resulting white solid was dissolved in 115 mL of concentrated HBr (115 mL). The acidic copper bromide solution was added to the diazonium salt suspension at 0°C. The resulting mixture was gradually heated to 100°C and then stirred at this temperature for 1 hour. Significant bubbling was observed. After this time, the reaction mixture was cooled and the pH was brought to 4-5 by adding sodium carbonate. EtOAc was added and the mixture was filtered. The filtrate was extracted with EtOAc (3x). The organic layer was dried with Na2SO4 and filtered to obtain 70.
2-Bromo-5-hydroxymethylphenol (71): Suspended in 4-bromo-3-hydroxybenzoic acid methyl ester 70 (6.97g, 30.2mmol) in dichloromethane (360mL) at 0°C Add DIBALH (100 mL, 1M toluene solution, 100 mmol) into the solution in batches. Significant gas evolution was observed. The resulting mixture was warmed to room temperature and stirred for 2.75 hours. After this time, the reaction mixture was quenched by adding saturated aqueous sodium potassium tartrate solution (600 mL) and saturated aqueous ammonium chloride solution (100 mL). The resulting biphasic mixture was stirred vigorously for 2 hours and then extracted with dichloromethane (3x). The organic layer was dried with Na2SO4 and filtered to obtain 71.
Main reference materials
[1] U.S. Pat. Appl. Publ., 20040097485, 20 May 2004

 微信扫一扫打赏
微信扫一扫打赏

