Background and Overview
2-Hydroxymethyl-5-bromobenzimidazole, also called (5-bromo-1H-benzimidazole-2-yl)methanol, is an organic synthesis intermediate and pharmaceutical intermediate that can be used in laboratories In the research and development process and chemical and pharmaceutical synthesis processes.
Preparation[1]
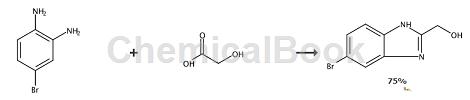
A mixture of 4N HCl (8.55mL, 51.3mmol), (2-amino-4-bromophenyl)amine (8g, 42.8mmol) and glycolic acid (4.88g, 64.2mmol) in water (3mL) Heat the microwave reactor at 120 °C for 1 h. Add NaOH (6N, 2mL). Solids precipitated. The solid was filtered and washed with diethyl ether to give the title compound (7.3 g, 32.2 mmol, 75% yield). (5-Bromo-1H-benzimidazol-2-yl)methanol, yield (7.3 g, 32.2 mmol, 75%).
Application [1]
For the synthesis of an epithelial sodium channel (ENaC) blocker:
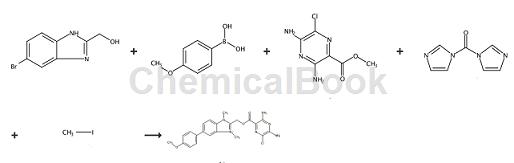
Sequence 1
Step 1
Synthesis of {5-[4-(methoxy)phenyl]-1H-benzimidazol-2-yl}methanol
A mixture of (5-bromo-1H-benzimidazol-2-yl)methanol (800mg, 3.52mmol), [4-(4-)methoxy)phenyl]boronic acid (803mg, 5.28mmol) , PdCl2 (dppf) (25.8 mg, 0.035 mmol) and sodium carbonate (1120 mg, 10.57 mmol) were dissolved in a mixture of 1,4-dioxane (1.500 mL) and water was added in a microwave vial (0.5mL). It was degassed for 5 minutes and then heated in a microwave reactor at 100°C for 10 minutes. The crude product was filtered through a PL-Thiol MP SPE+ column. The solvent was then concentrated to give the title compound (793 mg, 3.12 mmol, 89% yield). {5-[4-(Methoxy)phenyl]-1H-benzimidazol-2-yl}methanol, yield (793 mg, 3.12 mmol, 89%).
Sequence 2
Step 1
Synthesis of 3,5-diamino-6-chloro-2-pyrazinecarboxylic acid
A mixture of methyl 3,5-diamino-6-chloro-2-pyrazinecarboxylate (10g, 49.4mmol), 6N NaOH (9.87mL, 59.2mmol) and methanol (50mL) was stirred at room temperature. Spend the weekend. The solid precipitated from the solution. IN HCl was added to the mixture until pH was 2. The solid was filtered off, washed with diethyl ether, then collected and dried under high vacuum to give the title compound (9.35 g, 49.6 mmol, 100%) as a yellow solid. 3,5-diamino-6-chloro-2-pyrazinecarboxylic acid, yield (9.35 g, 49.6 mmol, 100%).
Step 2
Synthesis of 3-chloro-5-(1H-imidazol-1-ylcarbonyl)-2,6-pyrazinediamine
Combine 3,5-diamino-6-chloro-2-pyrazinecarboxylic acid (6g, 31.8mmol) and 1,1′-(oxomethanediyl)bis-1H-imidazole (6.7g, 41.4 mmol) in DMF (30 mL) was stirred at room temperature for 2 h. The crude product was filtered off and washed with DCM. The solid was collected and dried under high vacuum to give the title compound (7.5 g, 31.5 mmol, 99%) as a solid. 3-Chloro-5-(1H-imidazol-1-ylcarbonyl)-2,6-pyrazinediamine, yield (7.5 g, 31.5 mmol, 99%).
Step 3
Synthesis of {5-[4-(methoxy)phenyl]-1H-benzimidazol-2-yl}methyl 3,5-diamino-6-chloro-2-pyrazinecarboxylate
Mixture of 3-chloro-5-(1H-imidazol-1-one) (carbonyl)-2, 6-pyrazinediamine (450mg, 1.886mmol), {5-[4-(methoxy)phenyl]-1H-benzimidazol-2-yl}methanol (719mg, 2.83mmol) and TEA (0.789 mL, 5.66 mmol) in DMSO (4 mL) in a microwave vial was heated in a microwave reactor at 120 °C for 1 h. The crude product was purified by preparative HPLC (containing 0.1% NH4OH). The desired fractions were concentrated at 50°C under nitrogen flow to give the title compound (327 mg, 0.770 mmol, 40.8% yield). {5-[4-(methoxy)phenyl]-1H-benzimidazol-2-yl}methyl 3,5-diamino-6-chloro-2-pyrazinecarboxylate, yield (327 mg , 0.770mmol, 40.8%).
Step 4
2-({[(3,5-diamino-6-chloro-2-pyrazinyl)carbonyl]oxy}methyl)-1,3-dimethyl-6-[4-(methyl Synthesis of oxy)phenyl]-1H-benzimidazole-3-iodide
{5-[4-(Methoxy)phenyl]-1H-benzimidazol-2-yl}methyl 3,5-diamino-6-chloro-2-pyrazinecarboxylate (150mg A solution of a mixture of sodium hydride (15.53 mg, 0.388 mmol in a microwaveable vial) in DMSO (1.5 mL) was stirred at room temperature for 1 h, then methyl iodide (0.077 mL, 1.236 mmol) was added to the mixture. The resulting mixture was stirred at room temperature for 3 hours. The crude product was purified by automated flash chromatography to give the title compound (18.7 mg, 0.032 mmol, 9.12% yield). 2-({[(3,5-diamino-6-chloro-2-pyrazinyl)carbonyl]oxy}methyl)-1,3-dimethyl-6-[4-(methoxy) Phenyl]-1H-benzimidazole-3-iodide, yield (18.7 mg, 0.032 mmol, 9.12%).
Main reference materials
[1] PCT Int. Appl., 2011079087, 30 Jun 2011

 微信扫一扫打赏
微信扫一扫打赏

