Background and overview[1]
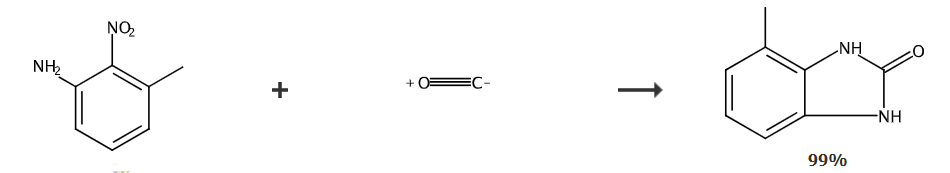
4-Methylbenzimidazolones are benzimidazolone derivatives. They are a class of fine chemicals with a wide range of uses. They can be used as important precursors for pigments and resins. The molecular structure contains (-CONH-) peptides. bonds, so most of them have biological activity, and some also have pharmacological activity.
Preparation[1-2]
Report 1,
Add 10 mmol of 3-methyl-2-nitroaniline (R in the general formula is an electron-donating substituent), 0.5 mmol of selenium powder, and 10 mmol of triethylamine into a stainless steel autoclave with a volume of 70 ml. and 10g toluene, seal, fill with 3.0Mpa carbon monoxide, put into an oil bath, quickly heat up to 150°C, react under insulation for 4 hours, cool to room temperature, open the kettle, combine the solid obtained by filtration with the solid obtained by concentrating the mother liquor, and dry , the product 4-methylbenzimidazolones was obtained by weighing. The crude reaction product was purified by recrystallization using ethanol as the solvent, and the added amount of the crude reaction product when it was completely dissolved when the solvent was close to the boiling point temperature. The purity is almost 100% and the yield is 99.3%.
Report 2,
Add 2mmol of 3-methyl-2-nitroaniline and 1mmol of NaHS (the molar ratio of o-phenylenediamine to NaHS is 1:2) into a 15ml polytetrafluoroethylene-lined reactor, and then add Use 1mL NMP as the reaction solvent, put a magnet in, tighten the reaction kettle, then fill it with 3MPa carbon dioxide, and stir the reaction at 90°C for 24 hours. After the reaction kettle was cooled to room temperature, it was extracted with ethyl acetate and washed with saturated brine. The organic layer was dried and the solvent was removed under reduced pressure to obtain a crude product; the crude product was separated by recrystallization or column chromatography (200-300 mesh silica gel, petroleum Ether and ethyl acetate were used as eluents) to obtain 4-methylbenzimidazolones.
References
[1][China invention, China invention authorization] CN01113966.8 A synthesis method of benzimidazolone and its derivatives
[2][Chinese invention] CN201710084842.3 A method for synthesizing benzothiazolones and 1,3-disubstituted urea derivatives using CO2 activation

 微信扫一扫打赏
微信扫一扫打赏

