Background and overview[1]
(5R)-3-(4-Bromo-3-fluorophenyl)-5-hydroxymethyloxazolidine- 2-Keto is an oxazolidinone compound. Oxazolidinones are a class of chemical compounds that find widespread use in drugs to treat and prevent medical conditions such as bacterial infections and atherosclerosis.
Preparation[1]
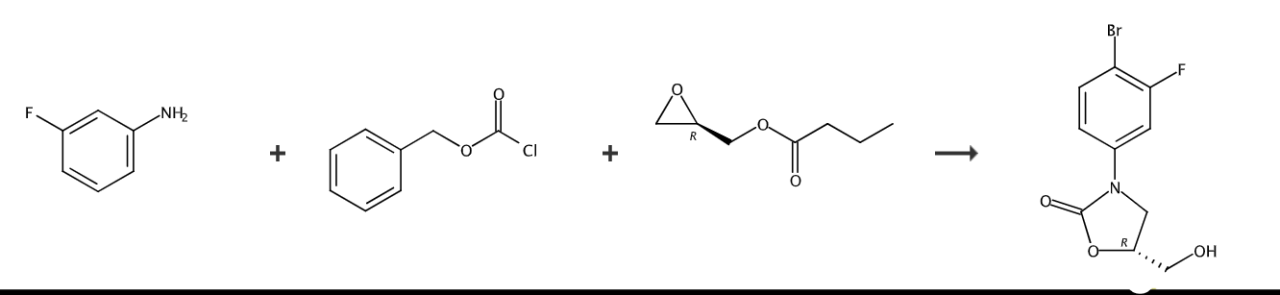
(1) Preparation of N-benzyloxyformyl-3-fluoroaniline:
Dissolve 100 grams of 3-fluoroaniline in 1 liters of tetrahydrofuran (THF), and 150 grams (1.8 mol) of sodium bicarbonate (NaHCO3) was added to the solution. After cooling to 0°C, 154 ml of N-benzomethoxymethane was added. Acid chloride (CbzCl) was slowly added to the solution to carry out the reaction. The reaction mixture was continued to react at 0°C for 2 hours under stirring conditions, and then the reaction system was extracted with 0.5 liters of ethyl acetate. After separation, the organic layer was washed with brine and washed with anhydrous magnesium sulfate (MgSO4) dried and concentrated in vacuo, and the residue was washed twice with n-hexane to obtain 132 g of the title compound as white crystals, with a yield of 85%.
Preparation of (2), (R)-3-(3-fluorophenyl)-2-oxo-5-oxazolidinylmethanol:
Place 132 grams N-Benzooxyformyl-3-fluoroaniline was dissolved in 1.3 liters of tetrahydrofuran, and the solution was cooled to -78°C. 370 ml of n-butyllithium (1.6 mol/L, n-hexane) was slowly added to the solution under a nitrogen atmosphere, and then stirred for 10 minutes. 84 ml of (R)-(-)-glycidyl butyrate was slowly added to the reaction mixture, stirred at the same temperature for 2 hours, and then reacted at room temperature for 24 hours. After the reaction was completed, ammonium chloride solution was added to the solution, and extracted with 0.5 liters of ethyl acetate at room temperature. The organic layer obtained was separated by washing with brine, dried over anhydrous magnesium sulfate, and concentrated in vacuo. The obtained residue was dissolved in 100 ml of ethyl acetate and washed with n-hexane to obtain white crystals, which were purified into 80 g of the title compound with a yield of 70%. 1H-NMR (DMSO-d6) δ7.85 (t, 1H), 7.58 (dd, 1H), 7.23 (dd, 1H), 4.69 (m, 1H), 4.02 (t, 1H), 3.80(dd, 1H), 3.60(br dd, 2H).
Preparation of (3), (5R)-3-(4-bromo-3-fluorophenyl)-5-hydroxymethyloxazolidin-2-one:
Dissolve 30 grams of (R)-3-(3-fluorophenyl)-2-oxo-5-oxazolidinylmethanol in 300 ml of acetonitrile, and add 46 grams of trifluoroacetic acid silver salt (CF3COOAg) and 30.5 g BrCl were added to the solution. After stirring at room temperature for 1 day, water was added to the solution and extracted with ethyl acetate. The separated organic layer was washed with brine and dehydrated. The residue was then filtered, concentrated in vacuo and dried, thereby obtaining 37.8 g of the title compound in 92% yield.
References
[1] [Invented in China, authorized by China] CN201510167731.X Preparation method of oxazolidinone compounds

 微信扫一扫打赏
微信扫一扫打赏

