Background and overview[1]
(2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl) Amino]-2-methylpentanoic acid is a key intermediate in the preparation of Sacubitril. Sacubitril and valsartan can form the compound drug Entresto at a ratio of 1:1. Entresto is a first-in-class medicine developed by Novartis to reduce the risk of cardiovascular death and hospitalization for heart failure and to reduce ejection fraction in patients with chronic heart failure (NYHA classes II-IV). Menstruation FDA approved for marketing.
Preparation[1]
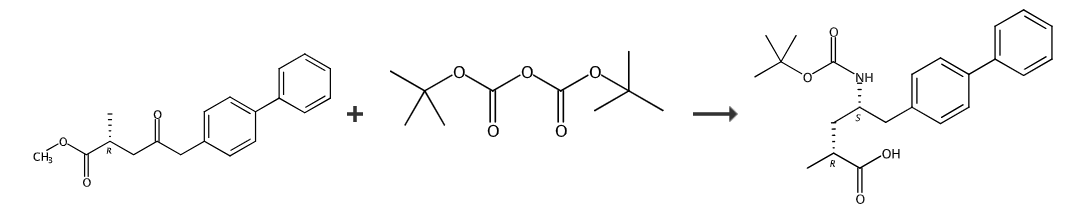
Step 1. Preparation method of (R)-2-methyl-4-oximino-5-(4-biphenyl)valeric acid:
To 500mL Add 20g (R)-2-methyl-4-oxo-5-(4-biphenyl)valeric acid, 8g hydroxylamine hydrochloride, 9.5g sodium acetate and anhydrous methanol into the three-necked flask, and heat until the reaction is complete. The reaction solution was concentrated, and a mixed solvent of water and THF (volume ratio 7:3) was added to the concentrated residue, stirred thoroughly and cooled with ice water, filtered, and dried to obtain an off-white solid. Weight 22.5g. Yield 98%. NMR data and chromatographic analysis results show that the product is a mixture of cis-trans configurations. Its characterization data are as follows: 1H-NMR (400MHz, CDCl3): δppm (Z/ E mixture) 10.78 (br, 1H), 7.54-7.59 (m, 4H), 7.42-7.45 (m, 2H), 7.278-7.36 (m, 3H), 6.25 (brs, 1H), 4.06 and 3.74 (d, 1H), 3.60-3.67(m, 1H), 2.94-2.96 and 2.74-2.81(m, 1H), 2.50-2.61(m, 1H), 2.27-2.43(dd, 1H). 13C-NMR (100MHz, CDCl3): δppm (main isomer) 180.11, 157.60, 140.71, 139.70, 129.55, 128.74, 127.42, 127.22, 126.99, 37.59, 37.36, 33.85, 17.58 δ ppm (minor isomer) 180.74, 158.54, 140.71, 138.91, 135.00, 129.55, 128.74, 127.42, 127.22, 126.99, 40.45, 36.23, 31.33, 1 7.44. MS(M+H): 298.1435, theoretical value: 298.1438
Step 2, (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl) Preparation method of amino]-2-methylpentanoic acid:
Add 2.0g (2R)-2-methyl-4-oximino-5-(4-biphenyl) into the hydrogenation kettle Methyl valerate, 30 mL methanol, 120 mg [RuCl(p-cymene)(R)-BINAP]Cl. Replace the air in the kettle with nitrogen, then replace the gas in the kettle with hydrogen, fill with hydrogen to 4.0MPa, and heat to 40 degrees for reaction. After the reaction is completed, cool to room temperature, slowly release the gas in the kettle, take out the reaction liquid, concentrate the reaction liquid to dryness, add 3M LiOH, heat and reflux the reaction, add di-tert-butyl dicarbonate after the reaction is completed, and use dicarbonate to react. Extract with methyl chloride, adjust the water phase to PH=2-4 with 6M hydrochloric acid, and extract with dichloromethane. After the extract is concentrated, a white solid is obtained with a yield of 80% and an optical purity of de=97.8%.
References
[1] [Chinese invention] CN201710697373.2 Preparation method of sacubitril and its intermediates

 微信扫一扫打赏
微信扫一扫打赏

