Background and Overview[1-3]
4-Chlorobenzaldehyde is mainly used in the manufacture of pharmaceutical raw materials and intermediates such as sedatives fenal, anaminobutyric acid, etc. It is also used in pesticides to manufacture chlorocinnamic aldehyde, weed-killing powder, etc.
Preparation[1]
The synthesis method of fenalol drug intermediate 4-chlorobenzaldehyde includes the following steps:
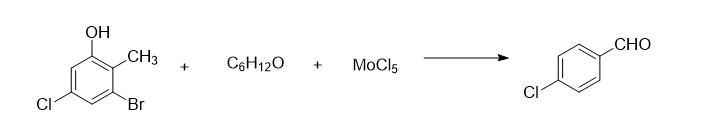
Add 2.5 mol of 2-methyl-3-bromo-5-chlorophenol and 3 mol of 80% 4-methyl-2-pentanone solution into the reaction vessel, control the stirring speed to 170 rpm, and increase Bring the temperature of the solution to 40°C, add 3.2 mol of molybdenum pentachloride powder three times, continue the reaction for 40 minutes after the addition, distill under reduced pressure at 1.32kPa, collect the fraction at 82°C, and add it to an 80% butyl glycolate solution. Wash 3 times in medium, wash 5 times in an 85% ethanedinitrile solution, and recrystallize in a 90% 2-methylbutyric acid solution to obtain 311.5g of crystalline 4-chlorobenzaldehyde, yield 89%.
Application[2-3]
CN201110089454.7 discloses a synthesis method of rimonabant hydrochloride: 4-chlorobenzaldehyde is condensed with nitroethane under alkali catalysis to obtain 1-p-chlorophenyl-2-nitropropene; D – Glucose reacts with 2,4-dichlorophenylhydrazine to obtain a phenylhydrazone compound; 1-p-chlorophenyl-2-nitropropene and a phenylhydrazone compound undergo cyclization reaction under alkaline conditions to obtain a pyrazole compound; with KMnO4 is a catalyst. The pyrazole compound is oxidized in an alkaline solution of NaIO4 to obtain a carboxylic acid compound; the carboxylic acid compound is chlorinated with a chlorine reagent and then reacts with N-aminopiperidine to form an amide and form a salt to obtain hydrochloric acid. Limonaban. The synthesis route of the invention is reasonable, the raw materials are cheap, the reaction conditions are mild, the total yield is high, the quality of the reaction intermediate is easy to control, the product has industrial production potential, and the product has high purity and stable quality.
CN201110432049.0 A method for preparing the broad-spectrum antibiotic chloramphenicol. The method uses 4-chlorobenzaldehyde and 2-nitroethanol as raw materials and synthesizes (1R, 2R)- in the presence of a chiral catalyst. 2-Nitro-1-(4-chlorophenyl)-1,3-propanediol is then catalytically hydrogenated to obtain (1R, 2R)-2-amino-1-(4-chlorophenyl)-1,3- Propylene glycol, the intermediate is nitro-substituted and dichloroacetylated to obtain chloramphenicol; the method provided by the invention can avoid the chiral separation and aluminum isopropoxide reduction commonly used in industry at present, and reduce three wastes, raw materials and reagents It is cheap and easy to obtain, uses p-chlorobenzaldehyde as raw material, has few synthesis steps and high yield, and is suitable for industrial production.
Main Reference Materials
[1] [Chinese invention] CN201710456138.6 Synthesis method of fenalol pharmaceutical intermediate 4-chlorobenzaldehyde
[2] CN201110089454.7 Synthesis method of rimonabant hydrochloride
[3] CN201110432049.0 A method for preparing chloramphenicol from 4-chlorobenzaldehyde

 微信扫一扫打赏
微信扫一扫打赏

