Background and overview[1][2]
1-Chlorobenzothiazide is a thiazide compound, which is widely used in various fields, such as tricyclic antidepressants and rubber curing agents.
Preparation[1-2]
Report 1.
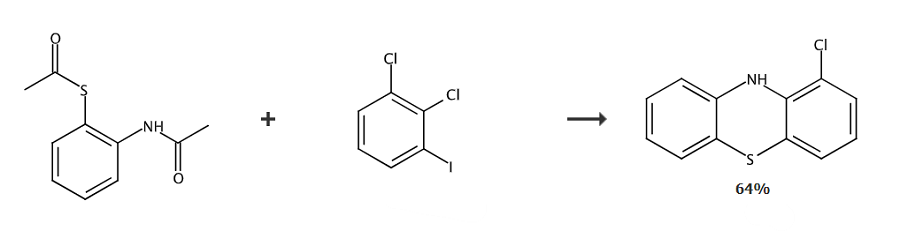
Place S-2-acetamidophenylsulfide (0.5 mmol), 2,3-dichloroiodobenzene (0.6 mmol), Cs2CO3 (2.0 mmol) and DMF (3 mL) were added to an oven-dried 25 mL test tube equipped with a stirring rod. Seal the test tube with a sleeve rubber stopper and drain and refill with argon for three cycles. The mixture was stirred at 130°C for 10 hours. After cooling to room temperature, the reaction mixture was quenched with ethyl acetate. Wash with water (20 mL) and extract three times with ethyl acetate (20 mL). The combined organic layers were dried over anhydrous MgSO4 and concentrated in vacuo. The residue was purified on a silica gel chromatography column.
Report 2,
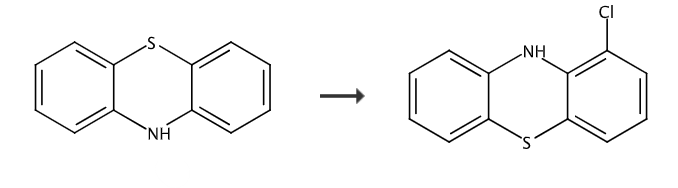
Cool the solution of phenothiazine (10.0g, 50.18mmol) in anhydrous THF (200mL) to -78°C, and add n-butyllithium (24.1mL, 60.2mmol, 2.5M hexane solution) dropwise. The mixture was stirred until a yellow precipitate formed and then allowed to warm to room temperature until a clear yellow solution was obtained. The solution was again cooled to -78°C and CO2 gas was bubbled through the mixture for 5 minutes. The resulting solution was allowed to warm to RT and the solvent was evaporated to give a residue. The residue was again dissolved in anhydrous THF (200 mL) and cooled to -78 °C, then t-BuLi (50 mL, 85 mmol, 1.7 M in pentane) was added dropwise. The resulting mixture was warmed to -20°C and stirred at this temperature for 2 hours. The reaction mixture was cooled again to -78°C and a solution of hexachloroethane (100 mmol) in THF (50 mL) was added dropwise. The mixture was stirred at this temperature for 1 hour, then allowed to warm to -20°C and stirred for 2 hours. The reaction mixture was quenched with ice-cold IN HCl and extracted with EtOAC. The combined organic layers were washed with brine, dried over MgSO4, filtered and evaporated to give a residue. The residue was purified by flash silica gel chromatography to give 1-chlorobenzothiazine as a beige solid.
Main reference materials
[1] Zhou Y , Zeng Q , Zhang L . Transition-metal-free synthesis of phenothiazines from S-2-acetamidophenyl ethanethioate and ortho-dihaloarenes[J]. Synthetic Communications.
[2] From PCT Int. Appl., 2011137447, 03 Nov 2011

 微信扫一扫打赏
微信扫一扫打赏

