Background and overview[1]
2-Hydroxy-4,5-dimethoxybenzoic acid phenyl ester is an important intermediate in the synthesis of 2-aminothiazole compounds. 2-Aminothiazole compounds are an important class of heterocyclic compounds in organic medicinal chemistry. They have a wide range of biological activities and can be used as local anesthetics. They have anticonvulsant, antiviral, antibacterial and insecticidal effects.
Preparation[1]
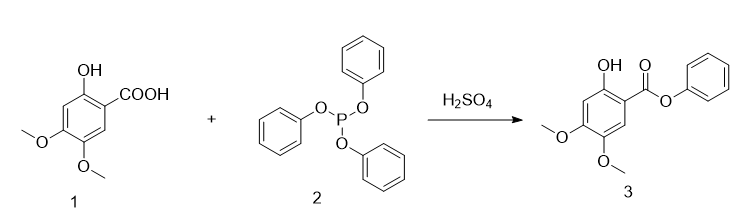
2-Hydroxy-4,5-dimethoxybenzoic acid phenyl ester is prepared as follows:
Under nitrogen protection, the reaction system was sequentially added to the reactor with 170g of toluene solvent, 266g (0.86mol) of triphenyl phosphite (compound 2), and 2-hydroxy-4,5-dimethoxybenzoic acid ( Compound 1) 170g (0.86mol) and 4.5ml of concentrated sulfuric acid (98wt%), heated to reflux (110°C) for 3 hours. After the reaction is completed, the temperature is lowered to 20°C, 566g of methanol is added dropwise to the reaction system, stir for 30 minutes, then 283g of water is added dropwise, stirred for 30 minutes, filtered, and washed with water to obtain 230g of crude 2-hydroxy-4,5-dimethoxybenzoic acid phenyl ester. , the crude product obtained was beaten and washed with ethanol, and after drying, 203g of pure 2-hydroxy-4,5-dimethoxybenzoic acid phenyl ester was obtained (yield 86.3%). The product structure of the obtained 2-hydroxy-4,5-dimethoxyphenyl benzoate contains seven groups of hydrogen atoms, and its chemical shift is consistent with the product structure. The single peak of the chemical shift at 3.78ppm is OCH3 at position 4 H peak, the single peak at 3.86ppm is the H peak of OCH3 at position 5, the single peak at 6.67ppm is the H peak of CH at position 3, and the quartet peak at 7.32ppm is the 1′, 3′ of phenyl formate at position 1 And the H peak of CH at position 5′, the single peak at 7.40ppm is the H peak of CH at position 6, and the triple peak at 7.51ppm is the H peak of CH at position 2′ and 4′ of phenyl formate at position 1, at 10.30ppm The single peak is the H peak of the 2-position OH.
Main reference materials
[1] CN201310466403.0 A kind of synthesis method of 2-hydroxy-4,5-dimethoxybenzoic acid phenyl ester

 微信扫一扫打赏
微信扫一扫打赏

