Background and overview[1]
2-(Difluoromethyl)benzaldehyde is an organic intermediate. There are reports in the literature that 2-(difluoromethyl)benzaldehyde can be prepared in one step from 1-bromo-2-difluoromethylbenzene and DMF. .
Preparation[1-2]
Report 1,
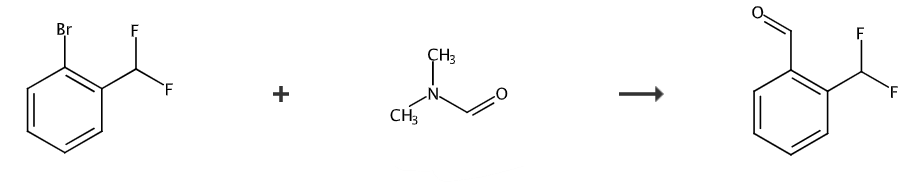
After dissolving 1-bromo-2-difluoromethylbenzene (3.23g) in tetrahydrofuran (65ml), the mixture was cooled to -78°C. n-Butyllithium (2.64M solution in hexane, 6.5ml) was then added and stirred for 30 minutes, N,N-dimethylformamide (2.4ml) was added and stirring was continued for 3 hours. A saturated aqueous ammonium chloride solution was added to the reaction mixture, and the mixture was diluted with water and extracted with ethyl acetate. The organic layer was washed with water and brine successively, and then dried over anhydrous magnesium sulfate. After filtration, the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography to obtain 2-(difluoromethyl)benzaldehyde (991 mg). 1H-NMR (400 MHz, CDCl3); δ 7.30-7.57 (m, 1H), 7.68-7.75 (m, 2H), 7.82-7.83 (m , 1H), 7.94-7.96 (m, 1H), 10.19 (s, 1H).
Report 2,
To a solution of methyl 2-(difluoromethyl)benzoate (700 mg, 3.76 mmol) in THF was added dropwise a 1 M solution of lithium aluminum hydride in THF (5.64 mL, 11.3 mmol) at room temperature. The reaction was then poured into ice water (100 mL) and diluted with ethyl acetate (200 mL) and 2N aqueous sodium hydroxide solution (100 mL). The organic phase was then separated, washed with brine (50 mL), dried over sodium sulfate and concentrated to give (2-(difluoromethyl)phenyl)methanol (480 mg) as an oil. 1HNMR (400 MHz, CDCl3) δ7.56 (d, 1H), 7.41 (m, 3H), 6.93 (t, 1H), 4.82 (s, 2H).
A mixture of (2-(difluoromethyl)phenyl)methanol (100 mg, 0.632 mmol) and manganese oxide (275 mg, 3.16 mmol) in dichloromethane was stirred at room temperature for twelve hours, then Stir at 45°C for 1 h. The reaction was filtered through celite and concentrated to give 2-(difluoromethyl)benzaldehyde as an oil (99 mg). 1H NMR (400 MHz, CDCl3) δ 10.2 (s, 1H), 7.94 (d, 1H), 7.81 (d, 1H), 7.70 (m, 2H), 6.93 (t, 1H).
Main reference materials
[1] From PCT Int. Appl., 2009101917, 20 Aug 2009
[2] From PCT Int. Appl., 2008041118, 10 Apr 2008

 微信扫一扫打赏
微信扫一扫打赏

