Background and overview[1]
(3R,4S)-3-Hydroxy-4-phenyl-2-azetidinone, referred to as (-)β-lactam, is a very potential chiral drug intermediate. There are two isomers, namely (3R, 4S) configuration and (3S, 4R). Among them, the derivative of paclitaxel side chain C-13 of the (3R, 4S) configuration isomer is the most useful for preparing cancer clinical chemotherapy. An important chiral precursor of docetaxel, one of the promising new drugs.
Preparation[1]
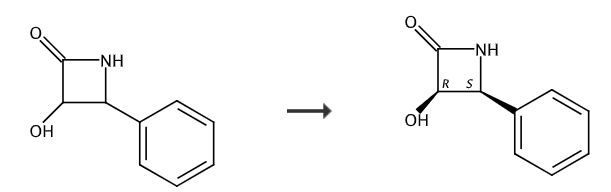
A soybean Bradyrhizobium whole-cell separation of 3-hydroxy-4-phenyl-2-azetidinone. The method includes the following steps:
(1) Strain selection: Bradyrhizobium japonicum is selected as the strain, and the strain preservation number is CGMCC No.1.2550; the strain is stored at -80°C;
(2) Fermentation broth preparation: Inoculate the soybean Bradyrhizobium strain into the rhizobia culture medium at a volume ratio of 0.1% to 0.5%, and culture it at 20°C to 30°C with shaking at 220 rpm for 96 to 168 hours. ;
Rhizobia liquid fermentation medium: 1.0g yeast extract, 10.0g mannitol, 200ml soil extract, 800ml distilled water, pH7.2;
The soil extract solution: 25g of soil, add 100ml of deionized water, cook at 105kPa for 1 hour, filter and add water to make up to the volume before cooking;
The specific preparation method is: dissolve yeast extract, mannitol, and soil extract in deionized water, adjust the pH value to 7.2 with NaOH, and sterilize at 121kPa for 20 minutes to obtain the rhizobia fermentation medium;
(3) Cell collection: Mix the cultured bacterial fermentation broth and phosphate buffer with a pH value of 7.0 thoroughly in a certain proportion to obtain a diluted fermentation broth; then centrifuge at 8000-11000 rpm for 10-15 minutes. Collect the bacterial precipitate; wash it again with a phosphate buffer with a pH value of 7.0, recover the wet bacterial cells after centrifugation, and collect the wet bacterial cells for use as a biocatalyst;
The phosphate buffer with a pH value of 7.0 consists of 0.05mol/l Na2HPO4 solution and 0.05mol/l NaH 2PO4 solution is mixed with a volume ratio of 7:3.
(4) Bioresolution reaction: Mix the wet bacterial cells with 1 to 10g/L racemic beta-lactam solution to obtain a bacterial mixture, in which 500ul of the reaction system contains 60 mg of wet bacterial cells ; The described bacterial mixture is shaken and cultured to obtain a reaction solution; the described racemic β-lactam solution is a racemic β-lactam and a general buffer, which is obtained after a certain period of ultrasonic treatment ;
The general buffers with different pH values are Tris50mM; Boric acid 50mM; citric acid 33mM; Na3PO4 50mM. Use HCl and NaOH to adjust pH to 3~12.
The temperature of the shaking culture is 25℃~50℃, the pH value is 5.0~10.0, the rotation speed is 200~220rpm, and the time is 3h~24h;
(5) Sample separation: Centrifuge the shaken bacterial liquid at a speed of 8000 to 12000 rpm for 5 to 10 minutes to obtain the supernatant, which is the (-) β-endogenous liquid. Amide sample.
(6) HPLC measurement: Take 500 μL of the (-) β-lactam sample and 200 μL of ethyl acetate for full shaking extraction to dissolve the target product in ethyl acetate; then take 10 μL of the described dissolved target product Inject a sample of ethyl acetate, use chiral HPLC to determine the content of (+) β-lactam and (-) β-lactam, and calculate the ee value and the yield of (-) β-lactam.
(7) The above-mentioned racemic β-lactam is 3-hydroxy-4-phenyl-2-azetidinone or 3-acetoxy-4-phenyl-2-aza Cyclobutanone.
(8) Corresponds to the (-)β-lactam obtained after splitting the whole cell in step (7): (3R,4S)-3-hydroxy-4-phenyl-2-azetidine Ketone or (3R,4S)3-acetoxy-4-phenyl-2-azetidinone.
(9) 3-Hydroxy-4-phenyl-2-azetidinone HPLC detection conditions: The column model is Chiralpark AS-H (250×4.6mm) of Daicel Company; use Daicel AS-H hand The linear column was used as the stationary phase, 100% acetonitrile, the flow rate was 0.6ml/min, and the detection wavelength was 210nm.
(10) 3-acetoxy-4-phenyl-2-azetidinone HPLC detection conditions: The column model is Chiralpark AS-H (250×4.6mm) of Daicel Company; Daicel AS- H chiral column was used as the stationary phase, 100% acetonitrile, flow rate 0.4ml/min, detection wavelength 210nm.
Main reference materials
[1] [Invented in China, authorized by Chinese invention] CN201410081161.8 A method for splitting racemic β-lactam using whole cells of Bradyrhizobium soybean [Authorized]

 微信扫一扫打赏
微信扫一扫打赏

