Background and overview[1]
(2R, 3S)-3-(Benzoylamino)-2-hydroxyphenylpropanoic acid ethyl ester is an important synthetic intermediate that can be used to synthesize the anti-cancer drug paclitaxel. The traditional synthesis method of (2R, 3S)-3-(benzoylamino)-2-hydroxyphenylpropionate ethyl ester mainly involves asymmetric dihydroxylation of α and β unsaturated esters, followed by azide substitution and reduction. be made of.
Preparation[1]
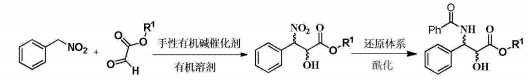
1) Add 0.786g (5.72mmol) nitrobenzyl, 5mL tetrahydrofuran and 0.731g (6.3mmol) isopropyl glyoxylate into a 25mL round-bottomed flask, add a magnet, and add 340mg to the system while stirring at room temperature (0.57mmol)1-(3,5-ditrifluoromethylphenyl)-3-[(6-methoxy-quinoline-4)-(5-vinyl-1-aza-bridged[ 2.2.2]Decan-2)-methyl]-thiourea, react at room temperature for 18 hours, evaporate the solvent, and perform column chromatography (petroleum ether: ethyl acetate 10~5:1) to obtain a colorless viscous oily substance of 3 -Nitro-3-phenyl-2-hydroxycarboxylic acid isopropyl ester 1.062g, yield 73.4%.
Main configuration product1HNMR (CDCl3, 500MHz): δ1.00 (d, J=6.5HZ, 3H), 1.20 (d, J= 6.5HZ, 3H), 4.83 (d, J=6.0HZ, 1H), 4.99-5.04 (m, 1H), 5.69 (d, J=6.0HZ, 1H), 7.40-7.47 (m, 3H), 7.51- 7.53(m,2H);28%ee.
2) Add 1.196g (5mmol) of chiral ethyl 3-nitro-3-phenyl-2-hydroxycarboxylate into a 50mL round-bottomed flask, add 25mL of acetic acid to it, and then add 1.625% of zinc powder to it g (25mmol), react at 35°C for 15 minutes, filter, wash the zinc powder with 50mL of ethyl acetate, wash the filtrate with 30mL of saturated sodium carbonate and 30mL of ammonia in sequence, dry the ethyl acetate layer over anhydrous sodium sulfate, and evaporate the ethyl acetate under reduced pressure ester; then add 10 mL of dry dichloromethane, 0.58 mL (5 mmol) of benzoyl chloride, and 0.66 mL (5 mmol) of triethylamine to it, stir at 20-30°C for 2 hours, evaporate the organic solvent under reduced pressure, and perform column chromatography. (Petroleum ether: ethyl acetate 10~5:1) The colorless viscous oil obtained was 1.25g of (2R, 3S)-3-(benzoylamino)-2-hydroxyphenylpropionic acid ethyl ester, yield 79.9 %.
1HNMR (CDCl3, 500MHz): δ1.12 (t, J=7.5HZ, 3H), 2.98 (d, 1H), 4.13-4.23 (m, 2H), 4.67 (m, 1H), 5.58 (dd, 1H), 7.06 (d, 1H), 7.20-7.56 (m, 8H), 7.80 (d, 2H); 27%ee.
Main reference materials
[1] CN201410455636.5 A method for synthesizing chiral 3-amino-3-phenyl-2-hydroxycarboxylate compounds

 微信扫一扫打赏
微信扫一扫打赏

