Background and overview[1]
(+)-Norfenefrine hydrochloride, also known as norfenefrine hydrochloride, scientific name m-hydroxyphenylethanolamine hydrochloride, English name NorfenefrineHydrochloride, is an adrenomimetic drug, a type of drug similar to epinephrine Drugs that excite nerves. Adrenergic receptors are divided into two types: alpha-receptors and beta-receptors. α-Receptors mainly exist on effector cells of blood vessels such as glands, skin, mucous membranes and internal organs. When α-receptors are excited, the main manifestations are the contraction of blood vessels in the skin, mucous membranes and visceral blood vessels, which increases peripheral resistance and increases blood pressure. Drugs that excite α-receptors are used clinically to increase blood pressure and fight shock. (+)-Norphenylephrine hydrochloride is clinically used to excite α-receptors, constrict blood vessels, increase peripheral resistance, and increase blood pressure.
Preparation[1]
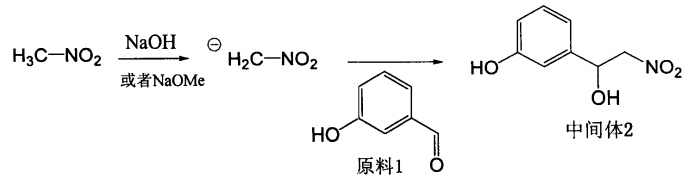
Add 5 ml of methanol into a 50 ml single-neck bottle, cool to 0°C, add 0.75g of metallic sodium, and stir until dissolved to obtain sodium methoxide. Slowly add 10 ml of nitromethane dropwise at 0°C. After the drop is completed, stir at 0°C for 15 seconds. Minutes, a light yellow negative ion suspension is formed, then dissolve 1.0g, 8.2mmol of m-hydroxybenzaldehyde in 10ml nitromethane, slowly add dropwise to the negative ion suspension, slowly rise to room temperature, stir overnight, add saturated ammonium chloride The reaction was terminated with the solution, and the reaction solution was extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated to obtain a light yellow oily substance, and purified by column chromatography to obtain a light yellow solid intermediate product 3- (1-Hydroxy-2-nitro)ethylphenol 1.104g, yield 73.6%, or 79.3% based on actual utilization of raw materials, and 72 mg of unreacted raw materials were recovered.
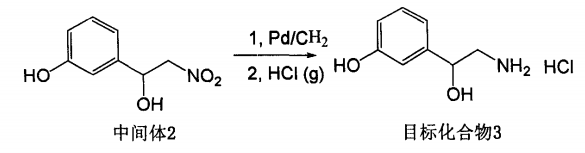
Add 200 mg of the intermediate product 3-(1-hydroxy-2-nitro)ethylphenol, 1.09 mmol, into a 25 ml single-neck bottle, dissolve it in 15 ml of anhydrous methanol, add 20 mg of palladium on carbon catalyst, and hydrogenate at room temperature and normal pressure. Catalytic hydrogenation, stirring for 10 hours, filtering through diatomaceous earth, and concentrating the filtrate to obtain 158 mg of crude product with a yield of 95%. Dissolve the crude product in dry ethyl acetate and pass in dry hydrogen chloride gas to form a salt to obtain the target compound (+)-detoluene. Folin hydrochloride.
References
[1][China invention, China invention authorization] CN200610070907.0 Preparation method of norphenylephrine hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

