Background and overview[1]
1,2-Diphenoxyethane (DPE) has attracted much attention as a new heat-sensitive material sensitizer and a potential polyolefin catalyst additive. There are only a few literature reports on the synthesis of 1,2-diphenoxyethane, which is mainly prepared by reacting phenol with 1,2-dichloroethane or ethylene glycol sulfonate as starting materials in alkaline water or ethanol medium. , the yield is reported to be up to 80%, but the product contains many impurities, and the synthesized crude product needs to be purified by high-temperature distillation (collection temperature is around 200°C, vacuum degree is below 700Pa). This process consumes a lot of energy, and due to the high freezing point of the product, it is easy to block the pipeline, placing high demands on the equipment. There are also reports on the synthesis of 1,2-diphenoxyethane using 1,2-dibromoethane and phenol as raw materials and ethanol as the solvent. However, the yield is only 30%. In the reaction, 1,2-dibromoethane It is prone to side reactions and is converted into vinyl bromide, resulting in low yield.
Preparation[1]
The preparation method of 1,2-diphenoxyethane (DPE) is as follows:
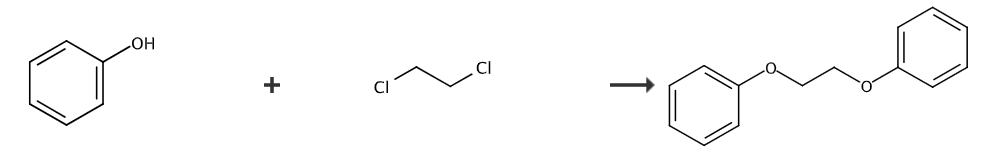
(1) Add 0.1 mol/L calcium hydroxide aqueous solution, toluene, and carbon tetrachloride into the high-pressure reaction kettle. The molar ratio of calcium hydroxide aqueous solution to toluene is 1:1, and heat at 70°C. Carry out hydrothermal reaction for 1 hour, and then place it in an oven to heat for 1 hour at 80°C;
(2) Add 1,2-dichloroethane dropwise to the reaction solution at 90°C for 1 hour at a rate of 2 seconds/drop while stirring to obtain the precursor 1,2-diphenoxyethane;
p>
(3) Place the precursor in a muffle furnace, heat it to 200°C and keep it for 5 hours. After the reaction is completed, cool the product to room temperature and take it out, grind it to obtain 1,2-diphenoxyethane, 1,2- The yield of diphenoxyethane was 86% and the purity was 97%.
References
[1]CN201910388002.5 A preparation method of 1,2-diphenoxyethane

 微信扫一扫打赏
微信扫一扫打赏

