Background and overview[1]
2-Fluoro-6-nitrotoluene can be used as a pharmaceutical synthesis intermediate, such as the preparation of fluorine-substituted indole compounds. Indole series products have various important biological activities, so the research and development of indole series products , has always been highly valued by the pharmaceutical industry. Especially in recent years, domestic and foreign journals and patent documents have often reported on indole series products and their preparation methods. By using cheap 2-fluoro-6-nitrotoluene as raw material, Enamine is generated through the condensation reaction with N,N-dimethylacetamide (DMA), and then the target compound 4-fluoroindole is obtained through reduction and ring closure.
Apply[1]
2-Fluoro-6-nitrotoluene can be used to prepare 4-fluoroindole, the steps are as follows:
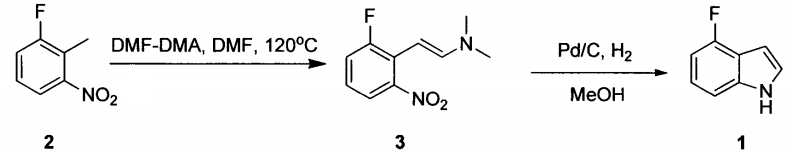
1. Preparation of compound 3:
In a 50 mL flask, add 2-fluoro-6-nitrotoluene (3.1g, 20mmol) and DMF (15 mL), and add DMF-DMA (4.76g, 40mol) with stirring. The temperature was raised to reflux and the reaction was carried out for 20 hours. HPLC monitored the completion of the reaction and then cooled to room temperature. The reaction was evaporated to dryness to obtain compound 3 (4.2g), with a yield of 100%.
2. Preparation of compound 1 (4-fluoroindole):
Place compound 3 (2.1g, 10mol) in a 50mL reaction flask, add methanol (10mL), add palladium carbon (5%, 300mg) with stirring at room temperature, and heat up to react with hydrogen gas overnight. After TLC monitoring, the reaction was terminated, filtered, and the solvent was evaporated under reduced pressure. The residue was purified with a silica gel column to obtain compound 1, 4-fluoroindole (0.5g), yield: 37%.
Preparation[2]
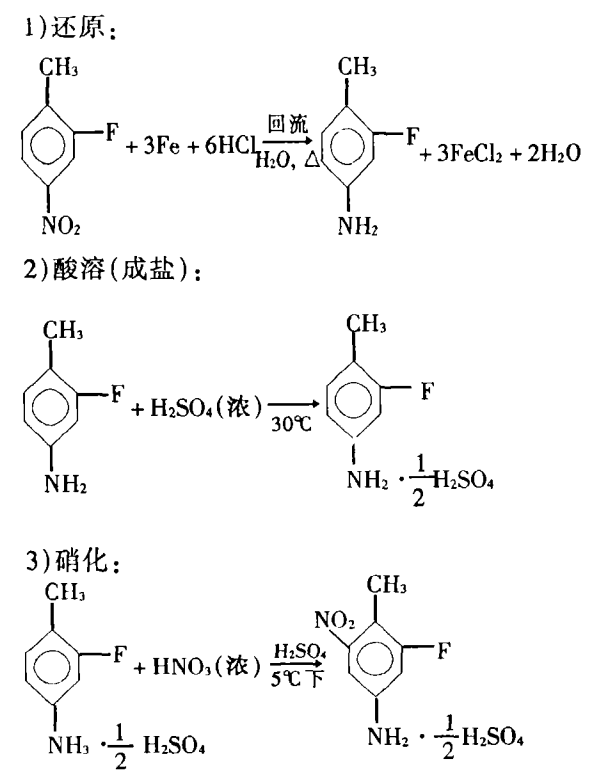
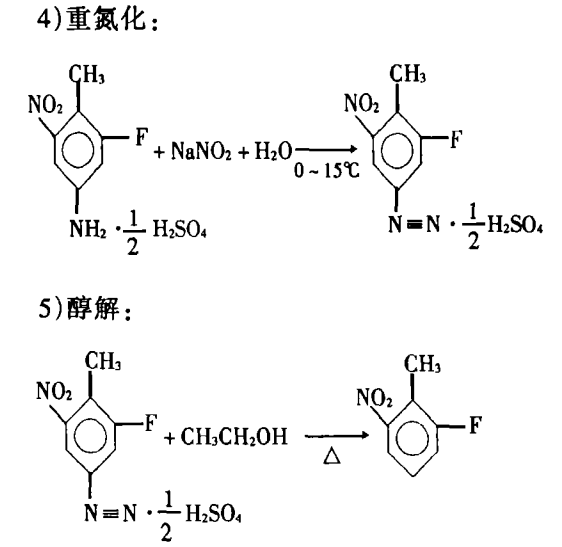
Using 2-fluoro-4-nitrotoluene as raw material, 2-fluoro-6 with a content of 98% can be obtained through reduction, salt formation (acid dissolution), nitration, diazotization, alcoholysis and steam distillation. -Nitrotoluene. The reaction equation is as follows:
References
[1] CN201210153837.0 A preparation method of 4-fluoroindole
[2] Ma Zhijun, Weng Xingyuan, Xu Lihong. Research on the synthesis of 2-fluoro-4-nitrotoluene and 2-fluoro-6-nitrotoluene[J]. Organic Fluorine Industry, 2003(02):5-7 .

 微信扫一扫打赏
微信扫一扫打赏

