Background and overview[1]
2-Bromophenylboronic acid, also called o-bromophenylboronic acid, is an important intermediate for organic light-emitting diode (OLED) electronic chemicals.
Preparation[1]
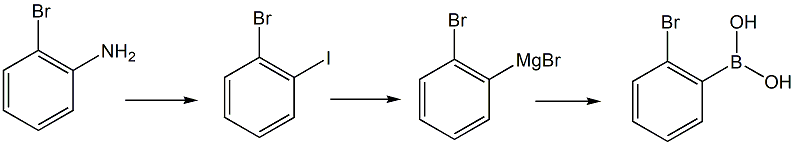
Using o-bromoaniline as raw material, the intermediate o-bromoiodobenzene is synthesized through diazotization and iodination, and then exchanged with isopropyl magnesium bromide for iodomagnesium to synthesize o-bromophenyl magnesium bromide, and then combined with trimethyl borate Methyl o-bromophenylborate is synthesized by substitution reaction, and then 2-bromophenylboronic acid is synthesized by hydrolysis.
1. Preparation of o-bromoiodobenzene
Add 172 g of o-bromoaniline and 350 mL of water to a 3 L four-necked flask equipped with a stirrer, condenser tube and thermometer. Slowly add 350 g of concentrated sulfuric acid and continue to raise the temperature to about 80°C until all the raw materials are dissolved. Cool to – At 10°C, add dropwise a solution of 75.9 g sodium nitrite dissolved in 150 mL of water. Complete the dripping in about 1.5 hours. React at about -5°C for 2 hours. Add 3 g of urea to decompose excess nitrous acid. Add 500 mL of dichloroethane, and dropwise add a solution of 176 g of potassium iodide dissolved in 200 mL of water. After the drops are completed, keep it at -10°C for 2 h, remove from the ice bath, and let it naturally rise to room temperature and stir overnight. Then stop stirring and separate into static layers. Wash the oil layer with sodium bisulfite solution until it turns orange, wash with sodium bicarbonate solution, and dry with 30 g of anhydrous magnesium sulfate. Distill under reduced pressure, and collect 235 g of the fraction at about 100°C (vacuum 2 mmHg), with a yield of 83% (HPLC purity 99.5%).
Synthesis of 2, 2-bromophenylboronic acid
Add 7.2 g of magnesium powder to a 500 mL four-neck bottle equipped with a stirrer, condenser tube and thermometer, heat to 80 ℃ under nitrogen, cool slightly, add 1 small grain of iodine and a small amount of isopropane bromide solution. After the reaction is initiated, add dropwise a solution of 37.5g of isopropyl bromide in 200 mL of tetrahydrofuran. The dripping is completed at about 65°C for about 1 hour, and the reaction is maintained at room temperature for 2 hours to prepare an isopropylmagnesium bromide solution. In a 500 mL four-neck bottle, add a solution of 43.1 g o-bromoiodobenzene and 80 mL tetrahydrofuran, seal it with nitrogen, cool it to about -10°C, and add dropwise the prepared isopropylmagnesium bromide solution until it is completed in about 1.5 hours. , incubate for 2 h, cool to about -25°C, quickly add 39.6g of trimethyl borate and 40 mL of tetrahydrofuran mixture dropwise, complete the addition in about 10 min, incubate for 1.5 h, remove from the ice bath and naturally raise to room temperature and stir overnight. Add dropwise a mixture of 35 g concentrated hydrochloric acid and 100 mL water while stirring, hydrolyze at room temperature for 1 h, filter out the solid, evaporate the solvent under reduced pressure from the filtrate (40°C), add 80 mL petroleum ether for extraction, and mix the oil layer with 80 mL Mix with water, evaporate the solvent, cool to precipitate a solid, filter at 15°C, and dry to obtain 21.3 g of solid. Add the crude product to 80 mL petroleum ether, beat at about 100°C for 1.5 h, cool to 25°C, filter and dry to obtain 18.5 g of product, yield 59.3%, HPLC purity 99.6%. Melting point 113.1~113.6℃ (literature value 113℃); IR: 3262 cm-1 stretching vibration absorption of associated O-H, 1400 cm-1, 1590 cm-1 skeleton deformation vibration of benzene ring, 1356 cm-1, B-O Stretching vibration absorption, vibration of 627 cm-1, 732 cm-1B-C.
Apply[2]
CN201410142897 discloses a preparation method of 4-bromocarbazole, which belongs to the field of organic chemical synthesis. This is achieved by the following method: using 2-bromophenylboronic acid as raw material, DMSO solvent, palladium metal and organic phosphine ligands to catalyze the Suzuki reaction with o-chloronitrobenzene to obtain 2-bromo-2′-nitrobiphenyl ; Then 2-bromo-2′-nitrobiphenyl is synthesized as the product 4-bromocarbazole using triphenyl phosphite as the reducing agent. The reaction process has few side reactions, simple operation and high yield. The synthesized 4-bromocarbazole can be used in organic optoelectronic materials, medicine and other fields, and is an important intermediate for carbazole optoelectronic materials, medicines and pesticides.
Main reference materials
[1] Liu Bingwen, Gai Hongwei, Li Changyin. Research on the synthesis of o-bromophenylboronic acid [J]. Liaoning Chemical Industry, 2014, 43(11): 1385-1386+1395.
[2] [Chinese invention, Chinese invention authorization] CN201410142897.1 A preparation method of 4-bromocarbazole


 微信扫一扫打赏
微信扫一扫打赏

