Background and overview[1]
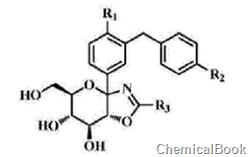
(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furyl]oxy]phenyl]methanone belongs to ketone organic compounds and is a pharmaceutical intermediate , can be used to prepare C-aryl glucoside SGLT2 inhibitors with the following general formula. SGLT2 is the major Na+/glucose cotransporter in the epithelial cells of the S1 segment of the proximal tubule and is responsible for 90% of glucose reabsorption in the kidney. SGLT2 inhibitors inhibit renal glucose reabsorption, allowing diabetic patients to reach normal plasma glucose levels through urinary glucose excretion, thereby increasing insulin sensitivity and delaying the development of diabetic complications.
Preparation[1]
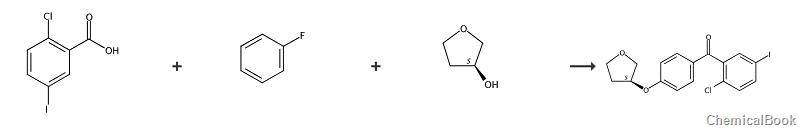
Step 1. Preparation of 5-iodo-2-chloro-4’-fluorobenzophenone
Add 5-iodo-2-chlorobenzoic acid (20.0g, 0.071mol) to 2.0M oxalyl chloride in dichloromethane (39ml, 0.078mol), stir to form a suspension, and then add 8 drops of DMF solution. , bubbles are generated. After 3 hours of reaction, the solution is clear and the reaction is basically complete. Place it on a rotary evaporator to spin the solvent dry, then add 15 ml of methylene chloride and spin the solvent dry. After spinning to dryness, add 30 ml of methylene chloride, stir, cool to 0-5°C, add fluorobenzene (7.1g, 0.074mol), add anhydrous aluminum trichloride (9.9g, 0.074mol) in batches, and control the temperature. Greater than 5°C, after the addition is completed, continue stirring at 4°C for 1 hour. TLC monitors that the reaction is basically complete. Place the reactant on a mixture of ice and water to quench the reaction, separate the organic phase, extract the aqueous phase with dichloromethane, and use 1 mol/ Wash twice with L hydrochloric acid, once with purified water, twice with 1 mol/L NaOH solution, twice with saturated sodium chloride, and dry over anhydrous sodium sulfate. Filtration was carried out, and the solvent was spin-dried to obtain an oily substance. After column chromatography, 20.1g (78.6%) of off-white solid was obtained.
Step 2, Preparation of (2-chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furyl]oxy]phenyl]methanone
Dissolve 5-iodo-2-chloro-4′-fluorobenzophenone (18.5g, 0.051mol) in 50ml tetrahydrofuran solution, then add (S)-3-hydroxytetrahydrofuran (4.9g, 0.056mol ) and stir, and finally add dropwise a solution of potassium tert-butoxide (6.9g, 0.061mol) dissolved in 30ml of tetrahydrofuran. Control the temperature at 16-25 degrees Celsius. After the dropwise addition is completed, stir and react at 20 degrees Celsius for 1 hour. TLC monitors that the reaction is basically complete. Slowly add purified water, stir for 30 minutes, let stand for liquid separation, and spin the organic phase to dryness. After column chromatography, 14.1 g (64.3%) of solid was obtained.
Main reference materials
[1][Chinese invention] CN201611142602.6 Bicyclic derivative of glucoside and its preparation method and use

 微信扫一扫打赏
微信扫一扫打赏

