Background and overview[1-3]
4-Aminophenylborate hydrochloride is an organic intermediate that can be prepared from dicatechol and 4-nitrobromobenzene as starting materials.

Preparation[1]
For the known reported 4-aminobenzene borate hydrochloride, 4-aminobromobenzene is usually used as the raw material. After the amino group is protected, n-butyllithium and borate are used under low temperature conditions of about -78°C. reaction, acidification and purification to obtain the product. This method has the following disadvantages: 1. The raw materials require amino protection and deprotection, which is cumbersome; 2. The reaction requires ultra-low temperature -78°C and harsh conditions. CN201210557943.5 provides a method for preparing 4-aminobenzene borate hydrochloride, which is characterized by:
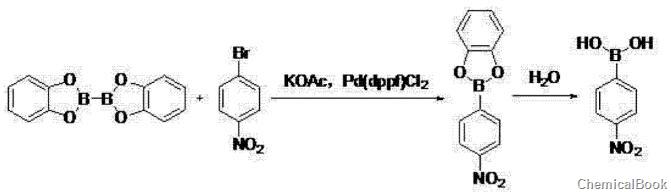
① In polar solvents, at a temperature of 80~100℃, dicatechol and 4-nitrobromobenzene, potassium acetate and Pd(dppf)Cl2 The reaction was carried out for 2 to 3 hours, then cooled to room temperature, filtered, and extracted with ethyl acetate. After concentrating the organic layer, the crude product was filtered by beating with a non-polar hydrocarbon solvent to obtain 4-nitrobenzene boric acid;
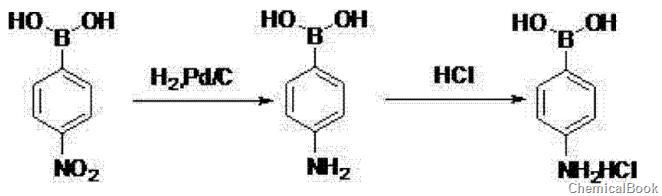
② 4-Nitrophenylboronic acid is hydrogenated in ethyl acetate, catalyzed by palladium on carbon, hydrogenated at 60~80℃, 0.8~1.0MPa for 6~8h, after the reaction is completed, filter at 15~25℃, 0~10℃ Add concentrated hydrochloric acid to the filtrate, filter to obtain a crude product, use acetone to beat the crude product, filter, and dry to obtain 4-aminobenzene borate hydrochloride.
The raw materials of this method have low toxicity and are easy to handle. At the same time, cryogenic temperatures are avoided, the reaction temperature is easy to reach, the conditions are mild, the yield is high, the process is safe and reliable, and it is suitable for industrial production.
Apply[2-3]
Polyimide is a polymer material with the highest heat resistance. Due to its excellent comprehensive properties, it has a wide range of applications in aviation, aerospace, electrical, machinery, chemical industry, microelectronics and other fields. In the 1960s, all countries were listing the research, development and utilization of polyimide as one of the most promising engineering plastics in the 21st century. Polyimide, due to its outstanding characteristics in performance and synthesis, whether as a structural material or as a functional material, its huge application prospects have been fully recognized.
CN201810453742.8 discloses a carbazole structure-containing diamine monomer and a polyimide synthesized therefrom. The present invention prepares a carbazole structure-containing diamine monomer through a one-step reaction of 3,6-dibromo-9-phenylcarbazole and 4-aminophenylborate hydrochloride, and the monomer undergoes a ring-opening reaction with an aromatic dianhydride. Polyamic acid is obtained, and then polyimide is obtained by heating to form a ring. The polyimide synthesis method of the present invention has a mild reaction and is easy to operate, which provides the possibility of industrialization. The polyimide material prepared by the invention shows excellent performance, especially significantly enhanced thermal stability, and has great potential application value and good application prospects in the fields of aerospace, electrical engineering, microelectronics and other fields.
CN201810866314.8 reports a preparation method of a new high-barrier polyimide material; the method of the present invention discloses a preparation method of a new high-barrier polyimide material. This method uses 2,7-dibromo Pyrene and 4-aminophenylborate hydrochloride are used to prepare a fused ring diamine monomer under alkaline conditions. The monomer diamine and the fused ring dianhydride monomer are reacted and polymerized to prepare a polyamic acid solution. Then, a water-carrying agent and a catalyst are added, and a polyimide slurry is prepared using a one-step method. After the slurry is formed into a film, the new high-barrier polyimide material can be obtained. This method introduces the fused ring structure into the main chain of the polyimide, which can make the polyimide molecular chains stack more tightly and increase the rigidity, restrict the movement of the molecular chains, reduce the free volume of the polymer, and make the polymer difficult to Form gas channels, thereby effectively improving the barrier properties of polyimide materials.
Main reference materials
[1] [Chinese invention] CN201210557943.5 Method for preparing 4-aminobenzene borate hydrochloride
[2] CN201810453742.8 A carbazole structure-containing diamine monomer and a polyimide synthesized therefrom
[3] CN201810866314.8 Preparation method of new high-barrier polyimide material

 微信扫一扫打赏
微信扫一扫打赏

