Background and overview[1]
4-[(4-hydroxy-2-pyrimidinyl)amino]benzonitrile can be used as an organic synthesis intermediate and pharmaceutical intermediate, and can be used in laboratory organic synthesis processes and chemical and pharmaceutical research and development processes.
Preparation[1]
Method 1: 4-[(4-hydroxy-2-pyrimidinyl)amino]benzonitrile is prepared as follows:
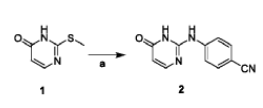
Weigh 2-(methylthio)pyrimidin-4(3H)-one (3g, 21mmol) and 4-aminobenzonitrile (2.99g, 25mmol) into a 50mL round-bottomed flask, protect with nitrogen, and slowly heat to 180℃, reaction 8h. After the reaction was cooled, 20 mL of acetonitrile was added for sonication, filtered, and the filter cake was washed with acetonitrile. TLC detected no 4-aminobenzonitrile residue. The filter cake was dried to obtain a light yellow solid, which is 4-((4-oxo-1,6 -Dihydropyrimidin-2-yl)amino)benzonitrile, yield 73.6%.
Method 2: 4-[(4-hydroxy-2-pyrimidinyl)amino]benzonitrile is prepared as follows:
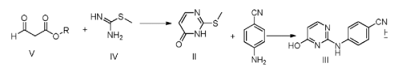
1) Synthesis of compounds of formula II
Dilute 1000g of ethyl formyl acetate (compound of Formula V where R is ethyl) with 5L of methanol, cool down, and slowly drop in 3L of ethanol solution of 510g of thiomethylisothiourea, and react at 5°C for 3 hours. Concentrate to dryness under reduced pressure. The residue is dissolved in hot water and acidified with acetic acid to pH=4. After cooling, the solid is precipitated and filtered. The filter cake is washed with water and recrystallized with water to obtain 764g of beige needle-like crystals. Yield: 94.9%, that is, Compounds of formula II;
2) Synthesis of compounds of formula III
Add 1kg of formula II compound and 692g of p-aminobenzoic acid into 8L of pyridine, heat to 110°C, react for 8h, cool to 0~5°C, stir for crystallization for 10h, filter, and wash with methanol to obtain 1206g of light yellow solid , yield, 97%, compound of formula III;
3) Synthesis of compounds of formula I
Add 1kg of compound III to 10L toluene, add 2.8kg of 40% hydrogen bromide acetic acid solution, heat to 100°C, react for 6 to 8 hours, distill toluene under reduced pressure, cool to room temperature, and pour into ice water. Neutralize with sodium hydroxide aqueous solution to neutrality, filter to obtain crude product, recrystallize with tetrahydrofuran to obtain 1211.7g of white solid, yield 93.5%, which is compound of formula I 4-[(4-hydroxy-2-pyrimidinyl)amino ] Benzonitrile, the purity detected by HPLC is 99.7%.
Main reference materials
[1] CN109369623-A substituted 1,2,3 triazole diarylpyrimidine derivative and its preparation method and application
[2] CN107162987-An industrial synthesis method and intermediate compound of rilpivirine

 微信扫一扫打赏
微信扫一扫打赏

