Overview[1]
As an important organic synthesis intermediate, o-fluorophenylhydrazine hydrochloride is widely used in dye and pharmaceutical manufacturing industries and has broad market prospects.
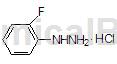
Preparation method[1]
A preparation method of o-fluorophenylhydrazine hydrochloride, the steps are as follows:
⑴ Diazotization: Mix 2-fluoroaniline and 37% concentrated hydrochloric acid and cool to 0~5°C. Add 35% sodium nitrite aqueous solution while stirring, keep the temperature between 0~5°C and react for 1~ 1.5 hours.
⑵Reduction: Add 37% concentrated hydrochloric acid, water and zinc powder to the reaction solution, keep the temperature between 15~20℃ until the reaction is complete, the reaction solution turns off-white, then add 20~30% sodium hydroxide The solution is adjusted to a pH of 10, and the mixture is incubated at 5° C. for 1 to 2 hours, crystals are precipitated, and filtered to obtain crude 2-fluorophenylhydrazine.
⑶Purification: Dissolve the crude 2-fluorophenylhydrazine in water, heat it to 60°C to completely dissolve it, then add an appropriate amount of activated carbon for decolorization for 20 minutes, hot filter to obtain a colorless filtrate, keep it at 5°C for 1 to 2 hours, and precipitate Crystals were filtered to obtain pure 2-fluorophenylhydrazine.
⑷Salt formation: Dissolve pure 2-fluorophenylhydrazine in 37% hydrochloric acid, stir at 60-70°C until the reaction solution precipitates and crystallize, cool to 20°C, filter, rinse the filter cake with acetone, and dry After that, the finished product of o-fluorophenylhydrazine hydrochloride is obtained.
The advantages and beneficial effects of this method are:
1. During the diazotization and reduction processes of this preparation method, concentrated hydrochloric acid is used to keep the reaction solution strongly acidic to ensure the smooth and complete progress of the reaction.
2. In the reduction process of this preparation method, zinc powder-concentrated hydrochloric acid is used as the reducing agent instead of sodium thiosulfate, sodium bisulfite, stannous chloride-hydrochloric acid, etc. It not only has good reduction performance and high yield, but also shortens the reaction time. Impurities such as zinc hydroxide generated after the reaction are easy to remove, making the product less impurities and high purity.
3. This preparation method uses acetone to rinse during the salt formation process, which not only improves the purity of the product, but also ensures the appearance of the product.
4. This preparation method is stable and reliable, easy to operate, has high product purity (its content is ≥99% as measured by high-performance liquid chromatography), and has a yield of ≥39%, which fully meets the market demand for o-fluorophenylhydrazine hydrochloride. .
Main reference materials
[1] [Chinese invention] CN201510578057.4 A preparation method of o-fluorophenylhydrazine hydrochloride

 微信扫一扫打赏
微信扫一扫打赏

