Background and overview[1]
Potassium tetrakis(4-tert-butylphenyl)borate can be used to prepare enantiopotential sensors based on potassium tetrakis(4-tert-butylphenyl)borate and can be used for identification detection between two enantiomers.
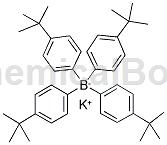
Apply[1]
CN201310686828 reports an enantiopotential sensor based on potassium tetrakis(4-tert-butylphenyl)borate, which is prepared by the following steps:
(1) Weigh 3 to 4 parts by weight of potassium tetrakis(4-tert-butylphenyl)borate, 64 to 66 parts of plasticizer and 32 to 33 parts of PVC, and dissolve them in 600 parts of tetrahydrofuran;
p>
(2) When each component is completely dissolved, pour the solution onto a glass plate to unfold naturally, and leave it at room temperature for 23 to 25 hours to obtain a PVC film;
(3) Stick the prepared PVC membrane to one end of the PVC hollow tube with a tetrahydrofuran solution with a PVC concentration of 8%, and leave it for 10 minutes. After the solvent has completely evaporated, pour KCl filling liquid into the tube, and insert the Ag/AgCl internal reference. Compared with the electrode, a PVC membrane electrode is obtained;
(4) The enantiomer potential sensor is composed of a PVC membrane electrode and a calomel electrode. The sensor expression is as follows:
Hg-Hg2Cl2︱KCl(satd.)|Test solution︱PVC membrane︱0.1mol·L-1KCl |AgCl-Ag.
The application of an enantiopotential sensor based on potassium tetrakis(4-tert-butylphenyl)borate in the detection of valine methyl ester specifically includes the following steps:
(1) First, soak the membrane electrode of the enantiopotential sensor based on potassium tetrakis(4-tert-butylphenyl)borate in 0.1mol·L-1 L-valerate prepared with buffer solution Activate in amino acid methyl ester solution for 1 day and set aside;
(2) Use buffer as solvent to prepare L-valine methyl ester solution and D-valine methyl ester standard solution with a concentration of 0.1mol·L-1, and then Dilute to 10-2, 10-3, 10-4, 10-5, 10-6mol·L-1To be tested;
(3) During detection, connect the membrane electrode and calomel electrode of the enantiomer potential sensor to the pH meter and insert them into the liquid to be measured at the same time;
(4) Wash the blank value with buffer until the electrode potential is constant, and then detect the potential response value of the L-valine methyl ester standard solution from the lowest concentration to the highest concentration;
(5) Then wash the blank value with buffer solution until the electrode potential is constant, and then detect the potential response value of the D-valine methyl ester standard solution from the lowest concentration to the highest concentration;
(6) Apply the detection results of steps (4) and (5), and use the valine methyl ester potential response value as the ordinate and the valine methyl ester concentration as the abscissa to draw a Nernst response curve, as Standard curve;
(7) Take the valine methyl ester to be tested, dilute it with buffer solution to a test sample with a concentration of 0.001mol·L-1, and detect its potential response value. Control the steps ( 6) Find the corresponding concentration value in the drawn standard curve, and then use the solution concentration calculation formula to obtain the accurate content of L-valine methyl ester in the valine methyl ester to be measured.
Compared with the existing technology, the advantages of the present invention are:
1. The analysis speed is fast, measurement can be achieved with a small amount of samples, low cost, economical and environmentally friendly;
2. Wide measurement range and high sensitivity, generally up to 4 to 6 concentrations.
3. The required equipment is simple, easy to operate, suitable for on-site measurement, and does not require too much equipment and maintenance costs.
Main reference materials
[1] [China invention, China invention authorization] CN201310686828.2 Enantiopotential sensor based on potassium tetrakis (4-tert-butylphenyl) borate and its application in the detection of valine methyl ester

 微信扫一扫打赏
微信扫一扫打赏

