Triphenylphosphine
【English name】Triphenylphine
[Molecular formula] C18H15P
[Molecular weight] 262.29
[CA registration number] 603-35-0
[Physical properties] White crystal, bp337°C/1.0 mmHg (133.322 Pa), mp 79-81°C, d 1.18g/cm3, soluble in most organic solvents, easily soluble in ethanol, benzene, and chloroform. Very soluble in ether, but insoluble in water.
【Preparation and Products】Sold by domestic and foreign reagent companies. It can be recrystallized from n-hexane, methanol or 95% ethanol and dried with CaSO4 or P2O5 at 65°C/1.0 mmHg (133.322 Pa) to obtain a pure solid.
【Precautions】It will irritate the human body under severe exposure to the sun, and may be neurotoxic if exposed for a long time. The reactivity of arylphosphine with oxygen is lower than that of benzyl and alkylphosphine, but the oxidation of triphenylphosphine by air is very obvious, forming triphenylphosphine oxide. Triphenylphosphine is not prone to fire and explosion, but it will generate toxic phosphine and POx fumes when heated and decomposed.
Triphenylphosphine is a quite commonly used reducing agent. In most cases, the reaction is driven by the formation of triphenylphosphine oxide, a thermodynamically favorable reaction. In addition, triphenylphosphine is widely used as a ligand for metal catalysts.
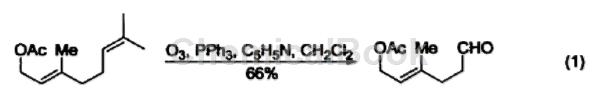
Deoxygenation reaction Triphenylphosphine is widely used in the reduction reaction of hydrogen peroxide or peroxide to produce alcohols, carbonyl compounds or epoxides. The main driving force for this type of reaction is the ability of triphenylphosphine to form strong P=O bonds with relatively weak O-O bonds (188-209 kJ/mol). For example, triphenylphosphine can be used to reductively decompose ozonides and selectively prepare ketones and aldehydes (Formula 1).
Reaction with azide Triphenylphosphine reacts with organic azide compounds to form iminophosphine (formula 2).
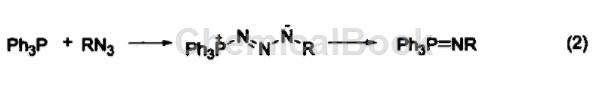
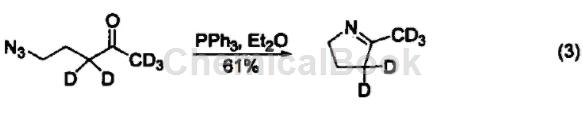
Iminophosphine is a relatively active nucleophile and easily reacts with electrophiles. For example: react with aldehydes and ketones to form imines and triphenylphosphine oxides. This reaction is similar to the Wittig reaction and is called the aza-Wittig reaction. The driving force of this reaction is also due to the generation of triphenylphosphine oxide (Equation 3).
Reaction with organic sulfides At room temperature, triphenylphosphine can convert episulfide compounds into alkenes (Formula 4).

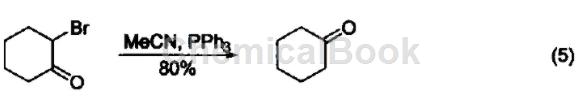
Dehalogenation reaction α-bromoketone reacts with triphenylphosphine to form ketone (Formula 5).
Reaction with organic epoxides. Refluxing in water and acetone solvents, triphenylphosphine can convert epoxy compounds into cyclic amine compounds with the participation of sodium azide (Formula 6).

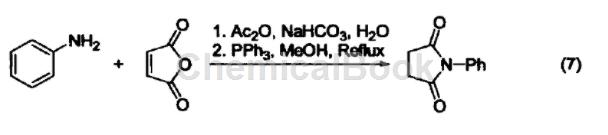
Preparation of substituted pyrrole: Aniline, furandione and triphenylphosphine react to form 1-phenyl-2,5-pyrroledione (Formula 7).
Used as a ligand for metal catalysts Triphenylphosphine can be used as a ligand to form metal catalysts with many transition metals. For example: Pd(PPh3)4 is an important catalyst and is often used to catalyze coupling reactions. These coupling reactions are important methods for building carbon-carbon bonds and are characterized by mild catalytic conditions. Another example: under the combined action of Pd(PPh3)4 and Ag2O, phenylboronic acid reacts directly with aromatic halogenated hydrocarbons to form biphenyl compounds. The yield of this reaction can reach 90% (Formula 8). In addition to phenylboronic acid and halogenated compounds, magnesium reagents, zinc reagents, tin reagents, silicon compounds, etc. can be used as substrates for coupling reactions.


 微信扫一扫打赏
微信扫一扫打赏

