Overview[1-2]
2-(4-Chlorobenzoyl)benzoic acid is an organic intermediate. It has been reported in the literature that it can be prepared in one step from benzoic acid and α-oxocarboxylic acid, or in one step from chlorobenzene and phthalic anhydride. get.
Preparation[1-2]
Method 1,
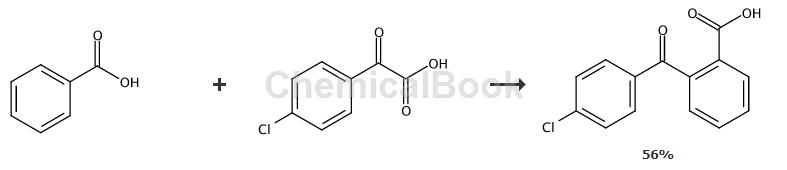
Add benzoic acid (1,0.2mmol), α-oxocarboxylic acid (2,0.6mmol), Pd (TFA) 2 (6.6mg) to a 20mL oven-dried pressure tube. , 0.02mmol), Ag2CO3 (0.5-0.6mmol) and DME (2.0mL). The tube was then sealed and stirred vigorously at 130-165°C for 24-48 hours. After cooling to room temperature, the reaction mixture was diluted with EtOAc (15 mL), filtered through a pad of Celite, and the filtrate was concentrated in vacuo. The residue was purified by silica gel flash chromatography (eluting with a gradient of 1% AcOH and 8 to 15% EtOAc in hexanes, v/v). 2-(4-Chlorobenzoyl)benzoic acid: light yellow solid, yield: 56%. 1H NMR (500MHz, CDCl3) δ: 7.35-7.39 (m, 3H), 7.58 (dt, J = 0.8, 7.7Hz, 1H), 7.63- 7.67 (m, 3H), 8.08 (d, J = 7.8Hz, 1H), 9.85 (brs, 1H); Ms (ESI): m/z = 259.0 [M-H+].
Method 2,
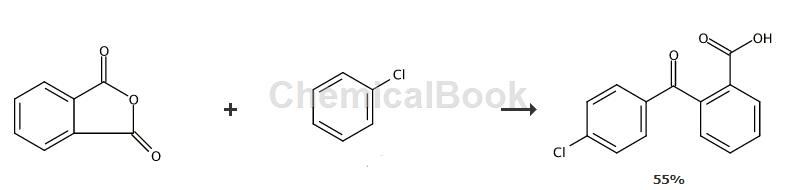
Place a mixture of chlorobenzene (20mmol), phthalic anhydride (2.36g; 16mmol) and anhydrous AlCl3 (4.36g, 32mmol) in an agate masher and pestle Grind thoroughly for 45 minutes. The reaction mixture was mixed with crushed ice and extracted with diethyl ether. The ether extract was washed with brine, dried over anhydrous MgSO4, and filtered. The filtrate was evaporated in vacuo; the residue was purified by silica gel column chromatography (Hexane/EtOAc, 8:2, v/v) to give the corresponding product.
Main reference materials
[1]Ma X , Huang H , Yang J , et al. Palladium-Catalyzed Decarboxylative N-3-ortho-C–H Acylation of 1,4-Disubstituted 1,2,3-Triazoles with α-Oxocarboxylic Acids[J]. Synthesis, 2018, 50(13):2567-2576.
[2]An Alternative Method for the Synthesis of γ-Lactones by Using Cesium Fluoride-Celite/Acetonitrile Combination[J]. Synthetic Communications, 2003, 33(19):3435-3453.

 微信扫一扫打赏
微信扫一扫打赏

