Background and overview[1]
1-Chloro-2-(4-ethoxybenzyl)-4-iodobenzene is a key intermediate in the preparation of dapagliflozin. Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor used to treat type 2 diabetes. It is a C-aryl glucoside compound with the chemical name: (2S, 3R, 4R, 5S, 6R)- 2-[3-(4-ethoxybenzyl)-4-chlorophenyl]-6-hydroxymethyltetrahydro-2H-pyran-3,4,5-triol. Dapagliflozin inhibits the reabsorption of blood sugar by inhibiting renal sodium-glucose cotransporter 2, thereby regulating blood sugar levels in the body; at the same time, it can significantly reduce the patient’s glycated hemoglobin level and weight. Dapagliflozin, jointly developed by Bristol-Myers Squibb and AstraZeneca, is the second SGLT2 inhibitor approved by the US FDA for the treatment of type 2 diabetes.
Preparation[1]

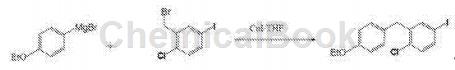
(1) Mix 5.0g 4-bromophenylene ether and 100mL tetrahydrofuran, stir until clear, set aside.
(2) Add 0.64g magnesium chips and 100mL tetrahydrofuran in sequence, start stirring to mix thoroughly, pour 20mL of tetrahydrofuran solution of 4-bromophenylethyl ether into it, heat to 45°C, when the specified temperature is reached, stop stirring and add 0.63 g elemental iodine particles trigger a reaction, and bubbles overflow from the bottom of the system. When a large number of bubbles overflow, start stirring and stir slowly until the system changes from dark red to colorless, clear and transparent, and the system temperature increases significantly.
(3) Add 0.63g of elemental iodine particles and return to normal speed, and begin to drip the remaining tetrahydrofuran solution of 4-bromophenylethyl ether. The system temperature is maintained at a slight reflux state, and the dripping is completed in 1-1.5 hours. After the dripping is completed, the micro-reflux reaction is maintained for 2 hours. Stop heating, cool down to 0°C, add 0.47g cuprous iodide to the reaction kettle, and further cool down to -20°C.
(4) Mix 8.24g 2-(bromomethyl)-4-iodochlorobenzene in 100mL tetrahydrofuran and set aside.
(5) When step (3) reaches the specified temperature -20°C, slowly add the tetrahydrofuran solution of 2-(bromomethyl)-4-iodochlorobenzene dropwise. There will be an obvious temperature rise process in the early and middle stages of the dropwise addition. Control the reaction system reaction at -20°C. The temperature rise was not obvious in the later stage of dripping, and the dripping was completed in 2 hours. After the dropwise addition is completed, keep warm and stir for 2 hours. Stop cooling, naturally warm to room temperature, and continue stirring for 12 hours. The system was quenched with 1N hydrochloric acid aqueous solution, and the organic phase was extracted with ethyl acetate, distilled, concentrated to a small volume until crystallized, filtered, and the filter cake was washed with pure water and dried by air blast. After weighing, the target product was obtained (the mass of the product 1-chloro-2-(4-ethoxybenzyl)-4-iodobenzene was 8.69g, the purity was 99.2%, and the yield was 93.11%).
Main reference materials
[1] [Invented in China] CN201811067934.1 Synthesis method of 1-chloro-2-(4-ethoxybenzyl)-4-iodobenzene

 微信扫一扫打赏
微信扫一扫打赏

