Overview[1]
Deuterated acetophenone is a deuterated reagent that can be prepared from benzene-d6 in two steps.
Preparation method[1]
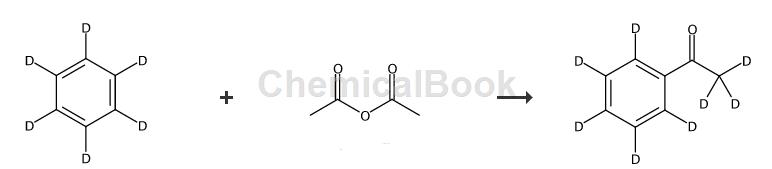
Step 1:
Preparation of acetophenone-2′,3′,4′,5′,6′-d5(2).
(Reference: Challacombe, K.; Leach, SE; Plackett, SJ; Meakins, GDJ Chem.Soc.Perkin Trans.1 1988,2213-2218.) In a 2L round bottom three-neck flask, a mechanical A stirrer, dropping funnel and reflux condenser connected to a gas absorption trap were used to treat the evolved hydrogen chloride. Benzene-d6(1) (100g, 1.19mol, 99.6atm%D) was placed in carbon disulfide (476mL). To this solution was added AlCl3 (356g, 2.67mol) under nitrogen at room temperature. The mixture was heated in the oil bath until gentle reflux began and acetic anhydride (89.8 mL, 0.952 mol) was added via dropping funnel over 1 hour. The reaction mixture was stirred at the same temperature for 1 hour and then poured into concentrated HCl. Add glacial hydrochloric acid (400mL). Separate the organic layer. Extract the aqueous layer with diethyl ether (400mL*2). Wash the combined organic layers with water (400mL *2) and saturate. water-based. NaHCO3 (400mL * 2) and brine (400mL), dried over MgSO4 and concentrated in vacuo. The residue was purified by distillation under reduced pressure to give 2 (103 g, 87% based on acetic anhydride). Acetophenone-2′, 3′, 4′, 5′, 6′-d5 (2), yield 103g, 87%
Step 2:
Preparation of acetophenone-d8(3).
To a solution of acetophenone 2 (104 g, 0.829 mol) in 1,4-dioxane (99 mL) was added a solution of sodium hydroxide (one pellet) in deuterium oxide (248 mL). After stirring at room temperature for 24 hours, the mixture was extracted with diethyl ether (300 mL×3). The organic layer was dried with MgSO4 and concentrated in vacuo to obtain crude product. The same operation is further repeated two more times. The final crude product was purified by distillation under reduced pressure to obtain 3 (90.1 g, 85%). Acetophenone-d8(3), yield 90.1g, 85%
Main reference materials
[1]Kobayashi Y , Hayashi N , Tan C H , et al. Toward the Creation of NMR Databases in Chiral Solvents for Assignments of Relative and Absolute Stereochemistry:? Proof of Concept[J]. Organic Letters , 2001, 3(14):2245-2248.

 微信扫一扫打赏
微信扫一扫打赏

