Background and overview[1]
P-hydroxyphenylpropionic acid is also called phloretinic acid. White to light yellow crystals. Relative molecular mass 166.18. Melting point 129~130℃. Boiling point 208~210℃ (1.867×103Pa). Insoluble in benzene and chloroform, soluble in ethanol, ether, ethyl acetate and hot water. The ortho position develops color when exposed to ferric chloride, but the para position does not develop color. It can be used as pharmaceutical raw materials, for preparing gastric medicine and as raw materials for pesticides.
Preparation[1]
① Use phenol as raw material to react with acrylonitrile to generate p-hydroxypropionitrile, which can then be hydrolyzed;
② Use p-hydroxybenzaldehyde as raw material, perform Perkin reaction with acetic anhydride in the presence of sodium acetate to generate p-hydroxyacrylic acid, and then hydrolyze it.
Application [2-5]
1. P-hydroxyphenylpropionic acid is used to synthesize 3-(4-hydroxyphenyl)propionamide
3-(4-Hydroxyphenyl)propionamide (Phloretamide) is a type of phenolic compound in apples that exhibits anti-aging, preventing skin sagging, and maintaining skin gloss. It is also produced as an anti-aging compound or as a component of anti-aging compounds. CN201410284642.9 provides a synthesis method of 3-(4-hydroxyphenyl)propionamide.
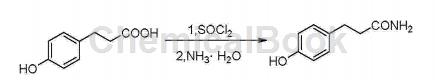
The specific steps are as follows:
①Add 15mL of thionyl chloride to 2g of p-hydroxyphenylpropionic acid, stir until clear, and detect the end point of the reaction by TLC;
② Spin off the thionyl chloride, add a small amount of acetonitrile to dissolve, slowly add dropwise to 20 mL of 25-28% ammonia water below 0°C, stir, and detect the end point of the reaction by TLC; purify.
TLC detection method in step ①: Take a sample, add a small amount of methanol to dissolve it, use the developer PE:EA=5:1 (mass ratio) to spot the silica gel plate, only one spot is generated, and no raw materials remain; TLC in step ② Detection method: Take a sample from a 1.5mL PC tube, add 1N HCl to acidify, extract with ethyl acetate, and use the developing agent DCM: MeOH = 10:1 (mass ratio) to spot on a silica gel plate. If there is no raw material left, the reaction is completed.
Purification method of step ②: After the reaction is completed, spin off the solvent, add a small amount of concentrated hydrochloric acid to the obtained solid, the solid turns from yellow to white, spin off the hydrochloric acid, spin to dryness, add excess ethyl acetate, heat to reflux, until Dissolve the solid, filter out the insoluble matter while hot, spin off most of the ethyl acetate, put it in ice, and precipitate the solid.
2. Used to prepare an ε-polylysine-p-hydroxyphenylpropionic acid antibacterial hydrogel dressing
CN201510289971.7 provides a hydrogel dressing with inherent antibacterial effect and good biocompatibility and biodegradability. Preparation method, which includes the following steps:
(1) Preparation of ε-polylysine-p-hydroxyphenylpropionic acid copolymer (EPL-HPA):
(1a) Dissolve p-hydroxyphenylpropionic acid (HPA) in a blended solvent of organic solvent and deionized water, stir and mix evenly;
(1b) Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide to the mixed system obtained in step (1a) Amine (NHS), activated in ice bath for 2 to 8 hours;
(1c) Add the ε-polylysine dissolved in deionized water to the system activated in step (1b), and react at room temperature for 10 to 20 hours;
(1d) Transfer the system obtained in step (1c) to a dialysis bag and place it in deionized water for dialysis for 3 to 7 days;
(1e) Freeze-dry the purified solution after dialysis in step (1d) to obtain ε-polylysine-p-hydroxyphenylpropionic acid copolymer.
3. Used to prepare a kind of dihydroavenoyl anthranilic acid D
Dihydroavenoyl anthranilic acid D has anti-irritation and anti-itching properties and is widely used in daily chemical products, such as baby nappy cream, shampoo, hair removal cream, etc. It is a substitute for oatanthramide and can Significantly improves clinical symptoms in patients with dry, itchy skin. CN201310413388.3 provides a synthesis method of dihydroavenanoyl anthranilic acid D. Includes the following steps:
(a) In a cold water bath at 5 to 18°C, using a chlorinated reagent as the solvent, p-hydroxyphenylpropionic acid and thionyl chloride are kept warm for 0.5 to 24 hours;
(b) Add methyl anthranilate dropwise to the reaction solution of step (a), then add inorganic base in batches, and stir at 8 to 35°C for 8 to 48 hours until the p-hydroxybenzoate is Propionic acid disappears, wherein, in terms of molar ratio, the p-hydroxyphenylpropionic acid: the methyl anthranilate ���The sulfoxide dichloride: the inorganic base=1:0.9~1:1~2:1~6;
(c) Add a concentrated solution of sodium hydroxide to the reaction solution in step (b), recover methylene chloride, add an alcohol solution for hydrolysis for 2 to 48 hours, the hydrolysis temperature is 18 to 60°C, and the pH value is controlled 10~14;
(d) After the hydrolysis is complete, stop the reaction, cool to room temperature, filter to remove insoluble matter, adjust the pH value to 4-7 with hydrochloric acid or phosphoric acid, if a white solid precipitates, filter the white solid, wash with water and dry to obtain the product .
4. P-hydroxyphenylpropionic acid is used to synthesize a universal hapten for dimethoxyphosphate pesticides
CN200710134520.1 synthesizes a universal hapten for dimethoxyphosphate pesticides. After coupling with protein, this substance can be used as a coating antigen in the enzyme-linked immunoassay for such pesticides. The coating antigen is used The difference in structure between the immunogen and the immunogen can further improve the detection sensitivity and reduce the detection limit.
Technical solution of the present invention: a synthesis method of a universal hapten for dimethoxyphosphate pesticides. The synthesis process consists of three steps: esterification, acylation and hydrolysis. First, esterification of p-hydroxyphenylpropionic acid and methanol occurs. reaction to esterify the carboxyl methyl; then the obtained methyl p-hydroxyphenylpropionate reacts with dimethoxyphosphoryl chloride to obtain dimethoxyphosphate; finally, the methyl ester formed by esterification in the first step is then reacted Hydrolyze and reduce the carboxyl group to obtain O,O-dimethyl-O-(4-propionophenyl) phosphate; this phosphate is coupled with egg white protein and has many residues as a dimethoxyphosphate pesticide. Coated antigen for ELISA analysis; the first step is to use p-hydroxyphenylpropionic acid as the raw material for methyl esterification and first protect the carboxyl group; the second step is to use the products of the previous step, methyl p-hydroxyphenylpropionate and dimethoxyphosphoryl chloride Acylation occurs to form a phosphate ester; the third step is to hydrolyze the methyl ester and deprotect the carboxyl group.
Main reference materials
[1] Practical Fine Chemical Dictionary
[2] CN201410284642.9 A synthesis method of 3-(4-hydroxyphenyl)propionamide
[3] CN201510289971.7 An ε-polylysine-p-hydroxyphenylpropionic acid antibacterial hydrogel dressing and its preparation method
[4] CN201310413388.3 A method for synthesizing dihydrooatenoyl anthranilic acid D
[5]CN200710134520.1 A synthesis method of universal hapten for dimethoxyphosphate pesticides

 微信扫一扫打赏
微信扫一扫打赏

