Background and overview[1]
2-Fluoro-5-iodobenzoic acid can be used as an intermediate for pharmaceutical and chemical synthesis. If 2-fluoro-5-iodobenzoic acid is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contact If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
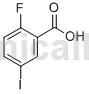
Preparation [1]
The preparation of 2-fluoro-5-iodobenzoic acid is as follows: Dissolve Pr2NH (16.80mL) in 200mL of newly distilled THF at -78°C, add n-butyllithium (68mL) dropwise, and maintain the reaction temperature Below -65℃. The reaction was stirred for 10 minutes, then a solution of 1-fluoro-4-iodobenzene (11.50 mL) in 10 mL THF was added over 20 minutes. The reaction was stirred at -78 °C for 90 min and then quickly inserted into diethyl ether (180 mL) and dry ice (~75 g). The reaction was stirred at room temperature overnight. Add 1N NaOH (100 mL) and water (200 mL) to the ether solution, and place the solution in a separatory funnel. Remove the water layer. Wash the organic layer with H2O (2 × 100 mL). Combine all aqueous fractions, cool in ice/H2O, and acidify to pH 2 with 6N HCl. The solution was then extracted with diethyl ether (2 x 200 mL). The ether fractions were combined, dried over Na2SO4, filtered and concentrated to give a light yellow solid. Dissolve the solid in a minimum amount of EtOAc on a steam bath and add hexane to effect recrystallization. After standing in the refrigerator overnight, the product was obtained as a crystalline white solid (15.44 g, 58%). The physical characteristics are as follows: m.p. Melting point 157-159℃;
Apply[1]
2-Fluoro-5-iodobenzoic acid can be used as an intermediate for pharmaceutical and chemical synthesis. Such as synthetic compounds:
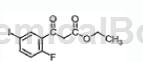
The specific steps are as follows: To a solution of 2-fluoro-5-iodobenzoic acid (4.03g) in 11 mL of freshly distilled THF, add 1,1′-carbonyldiimidazole) GDI (2.96g) in small portions. Violent gas evolution was observed. The reaction was stirred overnight.
In another flask, suspend ethyl malonate potassium salt (2.84 g) in 10 mL CH CN. To this solution was added chlorotrimethylsilane (2.15 mL) and the reaction was stirred at room temperature overnight. The latter reaction was cooled to 0°C and DBU (5.00 mL) was added dropwise, the reaction was stirred at 0°C for 3 hours, then the solution of CDI adduct was cannulated and the mixture was stirred at 0°C for 2 hours, After confirming complete conversion to product by TLC, the solution was quenched with water and 6N HCl (8 mL). The reaction mixture was partitioned with diethyl ether and the organic layer was washed with IN HCl then brine, dried over Na2SO4, filtered and concentrated to give a light orange oil. The oil was dissolved in EtOAc and adsorbed on silica. After purification by chromatography (eluent: 3% EtOAc/hexane), the desired product was obtained as a colorless oil, which crystallized after standing (2.51 g). Physical characteristics are as follows: m.p 54-56°C.
Main reference materials
[1] WO2001081318) 4-HYDROXYCINNOLINE-3-CARBOXYAMIDES AS ANTIVIRAL AGENTS

 微信扫一扫打赏
微信扫一扫打赏

