Background and Overview
4-Bromo-2-chlorobenzenesulfonamide is an amine derivative that can be used as a pharmaceutical intermediate.
Preparation[1]
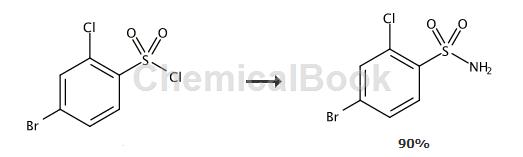
To a solution of 4-bromo-2-chlorobenzenesulfonyl chloride (2.50g, 8.45mmol) in THF (25ml) was added a solution of ammonia (25%, 6.3ml, 84.5mmol). The reaction mixture was stirred at room temperature for 4 hours. Add ethyl acetate (70 ml) and water (50 mL). The organic phase was separated, washed with hydrochloric acid (1M, 50ml) and brine (2 x 30ml) and dried over sodium sulfate. After evaporation, the product was obtained as a white solid (2.05 g, 90%).
Apply [2]
WO2009064848 reports that 4-bromo-2-chlorobenzenesulfonamide is used to prepare an NS5B polymerase inhibitor. NS5B is an RNA-dependent RNA polymerase (RdRp) that participates in HCV RNA replication. According to its structure, it is divided into two categories: nucleoside and non-nucleoside polymerase inhibitors. These two types of drugs have different mechanisms of action and can be used together.
Nucleoside polymerase inhibitors (NPI) are nucleoside analogs that have been modified by glycosylation or alkalinity. By simulating the natural substrate of the enzyme, they compete for the catalytic active site of NS5B and are inserted into the new In the synthesized nucleotide chain, the extension of the chain is terminated, thereby blocking the life cycle of HCV. The active site of NS5B is highly conserved and therefore effective against all HCV genotypes. WO2009064848 reports that compound 178 is an NS5B polymerase inhibitor and is synthesized as follows:
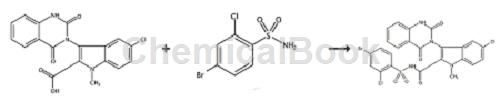
Compound 178 was prepared according to the method described in compound 98 below, starting from 5-chloro-3-(1,4-dihydro-2,4-dioxo-3(2H)-quinazolinyl )-1-Methyl-1H-indole-2-carboxylic acid to prepare compound 178, in which 4-bromo-2-chlorobenzenesulfonamide was used instead of methanesulfonamide. LCMS(M+H)622.3. Preparation of compound 98: 5-chloro-3-(1,4-dihydro-2,4-dioxo-3(2H)-quinazolinyl)-1-methyl-1H-indole-2 – To a solution of carboxylic acid (0.20 g, 0.41 mmol) in anhydrous THF (3 mL) and anhydrous DMF (1 mL) was added N,N’-carbonyldiimidazole (66 mg, 0.41 mmol). The reaction mixture was heated at 80°C overnight. The reaction mixture was cooled to room temperature and methanesulfonamide (39 mg, 0.41 mmol) was added. The reaction mixture was stirred at room temperature for 5 minutes and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (62 mg, 0.41 mmol) was added. The reaction mixture was stirred at room temperature overnight. Ethyl acetate (50 mL) was added and the organic layer was washed with 1N hydrochloric acid and brine. Dry the organic layer over sodium sulfate. The organic solvent was evaporated under reduced pressure. The crude product was purified by RP-HPLC.
Main reference materials
[1] PCT Int. Appl., 2009007015, 15 Jan 2009
[2] From PCT Int. Appl., 2009064848, 22 May 2009

 微信扫一扫打赏
微信扫一扫打赏

