Background and overview[1]
P-Toluenesulfonamide (PTS) is an extremely important intermediate for organic synthesis of drugs. It is often used in the synthesis of dozens of types of combined amisulfame, chloramphenicol, chloramphenicol‑T, thiamphenicol, etc. drug.
Preparation[1]
The existing mature production process of p-toluenesulfonamide uses a batch reactor, using p-toluenesulfonyl chloride as raw material, mixed with 20% ammonia water, adding 2.5 times the mass of water to p-toluenesulfonyl chloride, and heating to 55°C for 2 hours. , then raise the temperature to 65°C and keep it for 2 hours, then raise the temperature to 75°C and keep it for 2 hours, and finally raise the temperature to 95°C and keep it for 1 hour. After the reaction is completed, cool and filter to obtain crude solid product. After washing, filtering and drying, the product p-toluenesulfonamide is obtained. The reaction equation is as follows:

This reaction process will produce a large amount of ammonia nitrogen wastewater, and the yield is low. Intermittent reaction is labor intensive.
CN201210008930.2 proposes an industrial production method of p-toluenesulfonamide, which uses p-toluenesulfonyl chloride and ammonia to continuously react to produce p-toluenesulfonamide. This industrial production method of p-toluenesulfonamide uses p-toluenesulfonyl chloride and ammonia to react to generate p-toluenesulfonamide. The specific steps are:
a. Amination: The dichloromethane solution of p-toluenesulfonyl chloride is poured into the primary amination kettle, and enters the secondary amination kettle in an overflow manner through the side outlet of the primary amination kettle, and then passes through the secondary amination kettle. The side outlet of the amination kettle enters the third-stage amination kettle in the form of overflow, and then enters the fourth-stage amination kettle in the form of overflow through the side outlet of the third-stage amination kettle, and then enters through the lower outlet of the fourth-stage amination kettle. Centrifuge; on the other hand, ammonia gas enters from the tertiary amination kettle, passes through the submerged extension tube, contacts and reacts with p-toluenesulfonyl chloride, and the unreacted ammonia gas enters the secondary amination kettle through the gas phase pipeline and p-toluenesulfonyl chloride reacts, and the remaining ammonia gas enters the primary amination kettle through the gas phase pipeline to react with p-toluenesulfonyl chloride, and the remaining ammonia gas finally returns to the ammonia system;
b. Separation: The centrifuge will centrifuge the incoming material to separate out the p-toluenesulfonamide solid, and the remaining dichloromethane will enter the mother liquor collection tank;
c. Dissolution: The p-toluenesulfonamide solid is sent to the dissolving kettle through a conveyor belt, ethanol is added and heated to completely dissolve the p-toluenesulfonamide solid and become an ethanol solution of p-toluenesulfonamide;
d. Purification: The ethanol solution of p-toluenesulfonamide is filtered through a centrifuge. After the insoluble impurities are separated, it enters the decolorization kettle, mixed with activated carbon for decolorization, and then poured into a self-cleaning filter to separate the activated carbon. ;
e. Crystallization: Pour the ethanol solution of p-toluenesulfonamide obtained in the previous step into the crystallization kettle, cool the crystallization, and then use a centrifuge to separate the p-toluenesulfonamide solid from the ethanol;
f. Water washing: Send the p-toluenesulfonamide solid obtained in the previous step to the water washing kettle through the conveyor belt for water washing, and then enter the centrifuge from the discharge port at the bottom of the water washing kettle to separate the water to obtain the p-toluenesulfonamide solid .
This industrial production method of p-toluenesulfonamide adopts a multi-kettle series reaction method, and p-toluenesulfonyl chloride and ammonia gas are used in a solvent to perform a continuous amination reaction to produce amide in a countercurrent absorption manner. In order to achieve continuous production At the same time, it achieves the standard emission of ammonia in the tail gas, reduces environmental pollution, and reduces labor intensity. The use of solvents instead of water for the amination reaction eliminates hydrolysis side reactions, improves the reaction conversion rate, and greatly reduces COD emissions in wastewater.
Apply [2-3]
1. p-Toluenesulfonamide drugs are specifically used in the preparation of drugs for the treatment of head and neck squamous cell carcinoma, breast cancer, lung cancer, liver cancer, and malignant superficial solid tumors. Phase II clinical trials have shown that the p-toluenesulfonamide drug has strong killing power against tumors, has tumor-targeting functions, and has no obvious side effects on the human body. The benefits of PTS treatment are as follows: local and intratumoral injection before surgery can help prevent tumor metastasis and reduce tumor size, which in turn helps reduce the scope of surgery. Local injection of PTS after surgery may help to kill residual tumor cells, especially in patients with positive resection margins. PTS is used in combination with other chemotherapy or radiotherapy to prevent localizedlocal tumor recurrence or metastasis without adding additional toxic side effects. PTS can be administered intramuscularly, intraperitoneally or intravenously, and orally, and has the potential to be developed into an anticancer drug that can treat many different types of cancer.
Preparation examples of p-toluenesulfonamide drugs:
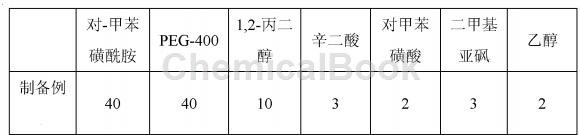
Mix the solvent auxiliary materials PEG-400, 1,2-propylene glycol, suberic acid, p-toluenesulfonic acid, dimethyl sulfoxide, and ethanol according to the adding proportion, heat to 80~110℃, stir, and make it into a Clear and transparent oily liquid. Gradually add p-toluenesulfonamide into the above-mentioned oily liquid and stir while adding. After it is fully dissolved, filter and cool to obtain p-toluenesulfonamide drug, in which each milliliter contains 345 mg of p-toluenesulfonamide.
Among them, the mass percentage of each component is as follows in Table 1:
2. Preparation of transparent high-concentration fluorescent chromogen
High-concentration fluorescent color essence is used to configure embossing and gravure printing inks for printing various packaging papers, transparent films, metal aluminum foils, gift papers, and is used in metals, plastics, glass, wood products, paper and other materials Surface coating, widely used. CN201310211518.5 provides a transparent high-concentration fluorescent color essence. In order to achieve the above object, the present invention adopts the following technical solution: transparent high-concentration fluorescent color essence, whose raw materials are composed of p-toluenesulfonamide, melamine, polyformaldehyde, hexylene glycol, and adipic acid, and the raw material ratio is: p-toluenesulfonamide Amide: melamine: polyformaldehyde: hexanediol: adipic acid = 1.0:0.35:0.45:0.1:0.1.
3. Used to prepare special fluorescent pigments for leather
CN201310211518.5 provides a special fluorescent pigment for leather. The following technical solution is adopted: special fluorescent pigment for leather. The raw materials are composed of p-toluenesulfonamide, melamine, formaldehyde, and polyurethane resin. The ratio is p-toluenesulfonamide: melamine: formaldehyde: polyurethane resin = 1.0:0.8:0.6:0.3.
Main reference materials
[1] CN201210008930.2 Industrial production method of p-toluenesulfonamide
[2] CN201110342765.X Application of p-toluenesulfonamide in the preparation of cancer therapeutic drugs
[3]CN201310211518.5 Transparent High Concentration Fluorescent Color Essence
[4] CN201310212183.9 Special fluorescent pigment for leather

 微信扫一扫打赏
微信扫一扫打赏

