Background and Overview
Methyl phenyl sulfide is a chemical intermediate.
Apply [1-2]
1. Used to synthesize a free radical photoinitiator
Photoinitiator is one of the indispensable components of UV curing materials, and it plays a decisive role in the sensitivity of the photocuring system. Since light-curing materials evaporate no solvent during curing, which greatly reduces environmental pollution, light-curing technology, as an environmentally friendly green technology, has developed vigorously in recent years.
CN201810101151.4 provides a method for synthesizing free radical photoinitiators. This method can effectively reduce production costs and increase product yield during the synthesis process. The technical solution adopted by the present invention is as follows: a synthesis method of a free radical photoinitiator, including the following steps: (1) under the action of aluminum trichloride, using methylphenyl sulfide as raw material, and 2-chloroiso Butyryl chloride undergoes Friedel-Crafts acylation reaction to produce intermediate A with a structure as shown in formula (Ⅰ); (2) Under the action of a base, intermediate A undergoes a cyclization reaction to produce a structure as shown in formula (Ⅱ) Intermediate B shown; (3) Intermediate B undergoes a ring-opening reaction with morpholine to obtain the target product with a structure shown in formula (III), which is the free radical photoinitiator. The method of the invention uses 2-chloroisobutyryl chloride and methylphenyl sulfide to carry out Friedel-Crafts acylation reaction, and then synthesizes the photoinitiator through steps such as cyclization and ring-opening. The obtained product has less impurities and a high yield. The process route is easy to operate, produces less three wastes, and is more environmentally friendly. The synthesis route of the free radical photoinitiator of the present invention is as follows:

2. Used in the synthesis of methylphenyl sulfoxide
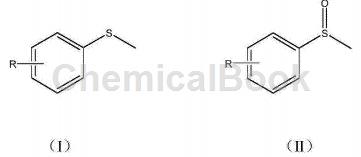
Sulfoxide is not only a commonly used solvent and extractant in organic synthesis experiments, but also widely used in the synthesis of various drugs. It is an important intermediate for medicines, pesticides and expensive materials. Choosing which catalyst can efficiently oxidize thioether to sulfoxide has always been a hot topic in the field of synthesis. Currently, the commonly used catalysts mainly include hydrogen peroxide, metal compounds and halogen compounds. CN201610819042.7 provides a simple and reasonable process, low cost, efficient and safe synthesis method of methyl sulfoxide compounds, using the SelectFluorTM oxidation method, in acetonitrile solvent and with the assistance of triethylamine, using SelectFluorTM as the oxidizing reagent, benzene Methyl sulfoxide compounds are synthesized using sulfide derivatives as raw materials. The technical solution to achieve the object of the present invention is as follows: a method for synthesizing methyl sulfoxide compounds, using the methylphenyl sulfide derivative shown in formula (I) as raw material, in a nitrogen atmosphere, using acetonitrile as the solvent, and SelectFluorTM (F-TEDA-BFI) is an oxidizing reagent. After reacting at room temperature for 10 minutes, add triethylamine (TEA) and react for another 10 minutes. After the reaction is completed, the reaction solution is separated and purified to obtain the methyl group shown in formula (II). Sulfoxide compounds.
Main reference materials
[1] CN201810101151.4 Synthesis method of free radical photoinitiator
[2] CN201610819042.7 A synthesis method of methylphenyl sulfoxide

 微信扫一扫打赏
微信扫一扫打赏

