Purpose and usage【1】
Benzil is an important organic chemical raw material and can be used in the synthesis of various drugs and photosensitizers, pesticides, printing inks for food packaging, and corrosion inhibitors for mild steel. Mainly used as a photoinitiator for UV assimilation resins and coatings. Within the effective wavelength range, its initiating efficiency, curing speed, thick film solidity, resistance to impurity interference and coordination with pigments are superior to benzoin ethers and benzophenones. . It can be applied to a variety of polymers, such as acrylic acid and its derivatives, styrenes and unsaturated resins. The dosage is 1% to 10% of the resin or monomer.
Synthesis
1. Crystallization method
This experiment will use the oxidation method to prepare benzoyl from an alpha-hydroxyketone, namely benzoin.
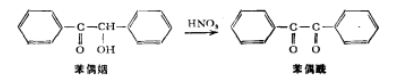
This oxidation reaction can be easily accomplished using a mild oxidizing agent such as Fehling’s solution (alkaline copper tartrate complex) or a pyridine solution of copper sulfate. In this experiment, nitric acid will be used to accomplish this oxidation.
Place 10 grams of benzoin and 50 ml of concentrated nitric acid in a 250 ml Erlenmeyer flask, heat the mixture on a steam bath in a fume hood, and shake the mixture from time to time until the nitrogen oxide gas (red) no longer until released (about 1 hour). Another way is, if using a trap device, heat the mixture on the laboratory table. Add sodium hydroxide to the trap to react with the nitrogen oxide gas. Pour the reaction mixture into 150 ml of cold tap water. Medium and stir vigorously until the oil completely crystallizes into a yellow solid. The crude benzil was vacuum filtered and washed thoroughly with cold water to remove nitric acid.
Recrystallize the product with 95% ethanol (4 ml/g). When the solution is cooled, scrape it with a stirring rod. If you do not do this, the solution will become supersaturated and the crystals formed will be yellow needles. Cool the mixture in an ice bath to complete the crystallization, vacuum filter the crystals using a Bachner funnel, and press the crystals with a clean glass stopper or cork to remove excess solvent.
Dry the product; or leave it in the air overnight, or place it in an oven at 75°C for about 10 minutes. Weigh the weight of benzil, calculate the percent yield, and then measure its melting point. The melting point of pure benzil is 95°C, the actual measured value is often lower than this value, and its range ranges from a low value of 84°C to a high value of 92°C. Products with melting points within this range are pure enough to be converted into diphenyl glycolic acid or tetraphenyl glycolic acid. Use of cyclopentadienone.
2. Green synthesis of benzil
In a three-neck flask equipped with a stirrer, reflux condenser and air conduit, add 10.8 g benzoin and 60 mL DMF (N, N-dimethylformamide). After dissolving, add Co(Salen ) Catalyst, KOH, heat in water bath, introduce air for oxidation, and use thin layer chromatography to track the reaction process. After completion, cool to room temperature (filter and remove the supported catalyst), adjust the pH value of the reaction solution to 3-4, add 150 mL of water to precipitate the solid, filter with suction, and wash with water. The crude product was recrystallized with 80% ethanol to obtain the oxidation product benzoyl, a yellow needle-like crystal, which was analyzed by HPLC and 1H NMR spectrum.
Production status
Benzil is a promising photosensitizer for UV-curable resins. In the past, Japan only imported some from other countries and sold them as reagents.
After Japan’s Kurogane Chemical Co., Ltd. successfully developed benzil in 1972, it developed uses as a photosensitizer for ultraviolet curable resins and has entered formal market development. In 1976, the company’s capacity was several hundred kilograms per month and plans were made to establish a formal production facility.
Purposes and requirements【3】
This product can be used as a photosensitizer for ultraviolet curable resin (UV resin).
The development of UV resin is very active, which is a measure taken to adapt to the restriction of the use of organic solvents in the fields of coatings and printing inks. The demand for UV resin is not yet very large, but it can significantly speed up the operation due to its rapid drying, and can also reduce the area of the curing equipment and shorten the operation line, so it has a promising future.
The photosensitizer for UV resin used to be benzophenone, benzoin and benzoin alkyl ether. Currently, the company that produces benzophenone in Japan is Katayama Chemical Company, and the company that produces benzoin and benzoin alkyl ether is Wako Pure Chemical Co., Ltd. Among them, benzoin alkyl ether has the largest demand, and the annual demand in the Japanese market is estimated to be about 6 tons.
The characteristic of benzoin is that it has a wide range of ultraviolet sensitization wavelengths. Benzoin is below 3400 A, benzoin alkyl ether is below 3900 A, and benzoyl is below 4,800 A. It can be sensitized in a wide wavelength range, so it can be used for curing thick film resins. This is Characteristics not found in previous UV resin photosensitizers. It also has no smell after curing, so it is suitable for printing ink for food packaging, etc.
However, the curing light intensity of this product is large, so it can be divided into two types: suitable and unsuitable according to the purpose of use. Compounds that can be sensitized for polymerization include: acrylic acid and its derivatives, methacrylic acid and its derivatives, styrenes, unsaturated polyesters, acryl compounds, itaconic acids, cinnamic acids, etc. The dosage varies depending on the resin and its use, but is generally about 1 to 10% of the resin. At this time, photosensitive accelerators such as amines must be added at the same time.
References
[1]Lu Bai�Editor-in-Chief, The Complete Book of Practical Industrial Additives, Chemical Industry Press, 1st Edition, August 2001, Page 200
[2] (U.S.) D.L. Pavia G.M. Lampman et al. Translated by Ding Xinteng, Introduction to Modern Organic Chemistry Experimental Technology, Science Press, January 1985, 1st edition, page 223
[3] Institute of Science and Technology Information, Ministry of Chemical Industry, World Fine Chemicals Handbook, Institute of Science and Technology Information, Ministry of Chemical Industry, 1st edition, December 1982, page 554

 微信扫一扫打赏
微信扫一扫打赏

