Background and overview[1-3]
Phenylalanine dehydrogenase LphenylalaninedehydrogenaseEC1.4.1.20, referred to as PheDH, is an oxidoreductase. Its positive reaction can catalyze the oxidative deamination of L-phenylalanine LPhe in the presence of NAD to generate phenylpyruvic acid NADH and The NH+4 reverse reaction can catalyze the synthesis of the corresponding amino acids from phenylpyruvate NADH and NH4+.
The catalytic properties of phenylalanine dehydrogenase make it a reagent enzyme for clinical detection of phenylalanine content in the blood of children with phenylketonuria (PKU). In addition, in the synthesis of chiral compounds, phenylalanine In addition to catalyzing phenylpyruvic acid and ammonia to form aromatic amino acids, acid dehydrogenase can also catalyze keto acids and ammonia to form the corresponding amino acids. Phenylalanine dehydrogenase has the ability to It has received widespread attention due to its huge commercial value and market potential.
Source of phenylalanine dehydrogenase[3]
Phenylalanine dehydrogenase was first discovered by German scholar Hummel et al. In 1984, while studying the phenylalanine metabolism of organisms, Hummel et al. found that it could be detected in the crude extract of Brevibacterium sp. strain Vll1 after cell disruption. To a dehydrogenase that can act on aromatic amino acids, namely phenylalanine dehydrogenase.
The reaction catalyzed by this enzyme is reversible. Its forward reaction must use L-phenylalanine as the substrate and NAD+ as the coenzyme to oxidatively deaminate to form phenylpyruvate. NADP+ cannot replace NAD+. Likewise, in its reverse reaction, reductive amination, in addition to phenylpyruvate, In addition, NADH and NH are needed to synthesize phenylalanine amino acid. NADH cannot replace L-glutamic acid with NADPH and cannot replace ammonia as the amino donor of phenylalanine. These indicate that the enzyme is L-phenylalanine dehydrogenase EC1.4.1. 20 It is different from the previously reported L-phenylalanine ammonia lyase phenylalanine ammonialyase, which is referred to as PALEC4.3.1.5, using astronine or glutamic acid as the amino donor.
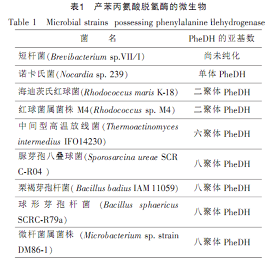
After that, some scholars successively isolated and screened PheDH-producing strains from other microorganisms. Most of the PheDH-producing organisms reported so far belong to aerobic Gram-positive bacteria or actinomycetes. Table 1. Engineering strains constructed through genetic engineering can also express and produce recombinant phenylalanine dehydrogenase. In 1987, Asano et al. cloned and expressed the phenylalanine dehydrogenase gene in E. coli and obtained a higher expression level of the enzyme in the culture medium. The total activity and specific activity can reach 6890UL and 7.05Umg respectively, and the properties of the purified recombinant PheDH enzyme are similar to those of natural PheDH enzyme.
Molecular weight of phenylalanine dehydrogenase of microbial origin [2]
The molecular weight of phenylalanine dehydrogenases derived from different strains varies greatly, about 42340kD. The reported phenylalanine dehydrogenases derived from microorganisms appear to be isoenzymes with different subunit numbers. According to phenylalanine dehydrogenase Depending on the number of subunits, they can be divided into four types: monomer, dimer, hexamer and octamer. Table 2: Although the molecular weights of the four types of phenylalanine dehydrogenase are different, The molecular weights of their subunits are very similar, about 3642kD.
Thermal stability of phenylalanine dehydrogenase[1]
The thermal stability of an enzyme often determines its application range. The thermal stability of the currently obtained phenylalanine dehydrogenases is quite different. The phenylalanine dehydrogenase only maintains 50% activity after being treated with 55 for 10 minutes. However, the phenylalanine dehydrogenase derived from phenylalanine dehydrogenase can still maintain 100% activity after being treated at 70°C for 1 hour. Villalonga et al. 13 modified the phenylalanine dehydrogenase derived from phenylalanine to make its thermal stability and optimal reaction temperature higher than those of the original benzene. The results of alanine dehydrogenase were increased by 8 and 10 respectively, which laid the foundation for obtaining higher thermostable phenylalanine dehydrogenase and its commercial application.
Applications of phenylalanine dehydrogenase[1]
Phenylketonuria, referred to as PKU, is an autosomal recessive genetic disease and is a key disease for newborn screening at home and abroad. Its clinical manifestations are an abnormal increase in the concentration of phenylalanine in the blood. If phenylketonuria is Timely diagnosis and treatment can completely avoid the intellectual impairment of children. The most commonly used method for neonatal PKU screening in early clinical practice is the bacterial inhibition assay.
However, this method is only a semi-quantitative experiment and cannot accurately measure the phenylalanine content in blood. In the 1990s, Wendel et al. described the development of a method for detecting phenylalanine content in plasma using phenylalanine dehydrogenase. A commercial phenylketonuria screening tool was produced for the microtiter plate test 1819.�Quantase20Schulze et al used Quantase to screen 423,773 newborns over a 6-year period. The results of this tool’s false positive screening for phenylketonuria were 0.23% and no false negatives, indicating that this screening tool fully complies with clinical medical testing requirements.
Problems and Outlook[3]
The application scope of phenylalanine dehydrogenase is getting wider and wider, but its enzyme source is relatively limited, and enzyme-producing strains have problems such as low yield and poor thermal stability of the enzyme. Therefore, use the diversity of microbial resources to screen more Excellent enzyme-producing strains are still the prerequisite for the development and application of phenylalanine dehydrogenase.
Using molecular evolution, site-directed mutation, genetic engineering and other means to obtain enzymes with better performance is the direction for the development of enzymes, a biological macromolecule. Research on phenylalanine dehydrogenase in the screening of enzyme-producing strains and molecular evolution. It is still in its infancy in China, so whether it is the screening of wild-type strains, the establishment of a mutant library based on existing reported strains, or the use of protein directed evolution to construct genetically engineered bacteria to produce phenylalanine dehydrogenase strains with excellent characteristics It is of great significance for the application of phenylalanine dehydrogenase in clinical testing and industry in my country to get rid of the dependence on imported phenylalanine dehydrogenase from abroad.
References
[1] He Guangzheng, Xu Shujing, Ju Jiansong. Study on the catalytic mechanism of alanine dehydrogenase[C]//Collection of abstracts of papers of the 11th China Enzyme Engineering Symposium. Wuhan, Hubei, China, 2017: 1.
[2] He Guangzheng, Xu Shujing, Ma Ning, etc. Overview of research on alanine dehydrogenase[J]. Biotechnology Bulletin, 2011 (12): 27-32.
[3] Xu Xiaohong, Wang Wei, Jia Xiaoming, etc. Research progress on phenylalanine dehydrogenase derived from microorganisms[J]. Science and Technology Bulletin, 2009, 25(03): 276-281+35.

 微信扫一扫打赏
微信扫一扫打赏

