Background and overview[1][2][3][4]
1,2,4-Trityl is a homologue of benzene, also called trimethylene or pseudocumene. It is mainly used in analytical reagents, organic synthesis and pharmaceutical industry. 1,2,4-trimethylbenzene has stable chemical properties. It is an important fine chemical raw material with high quality and high added value. It can be used to synthesize resins, dyes, medicines, plasticizers, etc. It is an ideal solvent for the production of hydrogen peroxide and an important raw material for the production of trimellitic anhydride, 3,4-dimethylbenzaldehyde, mesitylene and tetramethylbenzene. Generally speaking, my country has a large demand for such products. At present, the domestic annual production capacity of 1,2,4-trimethylbenzene is low, and most of it needs to rely on imports to make up for it.
Synthesis method[1][2]
Synthetic route 1: Isomerization of C9 aromatics
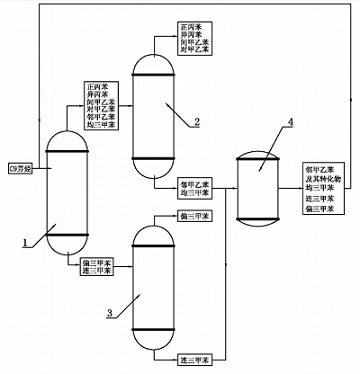
C9 aromatics are by-products during crude oil processing, mainly from the reforming unit and cracking unit of the refinery. C9 aromatics contain large amounts of 1,2,4-trimethylbenzene, mesitylene, trithionine, o-ethylbenzene, m-ethylbenzene, p-methylethylbenzene, n-propylbenzene and cumene, among which 1,2 , the content of 3-trimethylbenzene accounts for about 35% to 40%. The steps for producing 1,2,4-trimethylbenzene from C(aromatic hydrocarbon isomerization are as follows:
(1) The C9 aromatics produced by petroleum cracking with a purity of greater than or equal to 90% are treated in the light removal tower 1. The working temperature of the light removal tower 1 is controlled to be 166°C ~ 168°C, so that the boiling point is lower than 166 After the o-methylethylbenzene, m-methylethylbenzene, p-methylethylbenzene, mesitylene, n-propylbenzene and cumene at ℃ are vaporized and leave the light removal tower 1 from the top of the tower, they enter the first rectification tower 2; The 1,2,4-trimethylbenzene and trithionine retained at the bottom of the tower with a boiling point higher than 168°C are transported to the second distillation tower 3;
(2) In the second distillation tower 3, the distillation temperature is 169.5℃~175℃, 1,2,4-trimethylbenzene leaves from the top of the second distillation tower 3 and is collected. Trithionine remains at the bottom of distillation tower 3;
(3) In the first distillation tower 2, the distillation temperature is 162°C~164°C, and mesitylene and o-methylethylbenzene remain at the bottom of the tower;
(4) Send the bottom products of the above steps 2) and 3) into the closed isomerization reactor 4 at the same time to perform a catalytic reaction. The catalyst is arranged in the isomerization reactor 4 in the form of a fixed bed. , the reactant stream passes through the fixed bed in liquid form; the pressure inside the isomerization reactor is 0.2MPa, the reaction temperature is 260~270°C, and the bottom products of steps 2) and 3) continuously flow through the fixed bed, and under the action of the catalyst An isomerization reaction occurs under the conditions, causing part of the trimethylbenzene and mesitylene to structurally isomerize into 1,2,4-trimethylbenzene. At the same time, some o-methylethylbenzene is isomerized into p-methylethylbenzene and m-methylethylbenzene. ;
(5) Continuously input the reaction product obtained in step 4) into the light removal tower 1, and then send it to the second rectification tower 3 after light removal, where it will be rectified in the second rectification tower 3 to obtain 1,2 , 4-trimethylbenzene, the bottom product is sent to the isomerization reactor 4 again for recycling reaction. The single-pass conversion rate of this method can reach 45%. Its working principle diagram is as follows.
Synthetic route 2: Methylation of BTX aromatic hydrocarbons
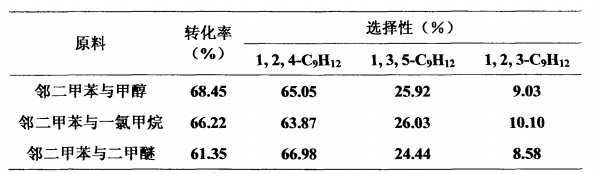
O-xylene is alkylated with methanol, dimethyl ether or methyl chloride to synthesize 1,2,4-trimethylbenzene, using a fixed bed continuous reaction. Reaction conditions: temperature 400°C, liquid mass space velocity 1h-1, o-xylene: methanol or methyl chloride (molar ratio) 1:1, o-xylene: dimethyl ether (molar ratio) 2:1, reaction time is 1 hour, normal pressure. The catalyst is HMCM-22 molecular sieve with SiO2Al2O3=50. The reaction properties of o-xylene alkylation to synthesize 1,2,4-trimethylbenzene are shown in the table below.
Application fields[3][4]
1.Production of trimellitic anhydride
The molecular structure of trimellitic anhydride (TMA) contains carboxylic acid and anhydride structures. It has the chemical properties of benzoic acid and phthalic anhydride. It can react with alcohol to form ester or polyester, react with alkali to form salt, and react with ammonia (amine) to form Amide-imide undergoes condensation reactions with hydrocarbons under the action of catalysts, making it useful in the production of PVC resin plasticizers, polyimide resin paints, water-soluble alkyd resins, epoxy resin curing agents, low-voltage and pulse power It has a wide range of applications such as impregnating agents for containers, film films, water treatment agents and surface activity. The steps for producing trimellitic anhydride by liquid-phase air staged oxidation of 1,2,4-trimethylbenzene are as follows:
(1) Put 400Kg 1,2,4-trimethylbenzene and 1000Kg acetic acid into the mixing kettle, then add 1.2Kg cobalt acetate and 1.2Kg tetrabromoethane. Control the mixing temperature to 60°C and start stirring to completely dissolve the catalyst
(2) Put the mixture into the oxidation tower, raise the temperature to 140°C and the pressure to 1.0Mpa, introduce air to initiate the stage oxidation reaction, control the oxidation temperature to 140-180°C, and the oxidation pressure to 0.4-1.0Mpa. After initiating for 30 minutes, add the prepared catalyst, including 0.8Kg of cobalt acetate, 0.8Kg of tetrabromoethane, and 0.8Kg of manganese acetate. Control the oxidation temperature to 180-300℃, the pressure to 1.0-3.0Ma, and adjust the air flow to reach 2000m3/h. Oxidation ends when the oxygen content of the exhaust gas reaches 18-20%
(3) The oxide material is dehydrated into crude anhydride, and the kettle temperature is controlled at 200-250°C. The dilute acetic acid produced during the anhydride formation process is sent to the distillation tower, concentrated and reused.
(4) The crude anhydride is distilled under reduced pressure to remove by-products to obtain 478Kg of finished partial anhydride. The kettle temperature is controlled at 200-280°C, and the vacuum degree is 10-20mmHg.
2. Synthesis of trimethylhydroquinone
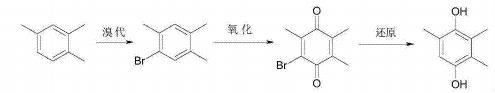
Trimethylhydroquinone is the main raw material for synthesizing vitamin E. Using 1,2,4-trimethylbenzene as the raw material, trimethylhydroquinone is finally prepared through bromination reaction, oxidation reaction, and reduction reaction. The reaction equation is as follows. This process has a short synthesis route and high yield. The bromine element can be completely recycled through recovery. It is a clean synthesis process that solves the shortage of cresol sources in the existing process and the serious pollution problems in the paraxylene process.
Main reference materials
[1] Cao Zhengguo, Zhou Aihua, Jing Xiaoping, Method for producing trimethylbenzene through isomerization of C9 aromatic hydrocarbons, CN 201110206928, application date 2011-07-22
[2] Wen Huaiyou, Sun Yimin, Duan Limin, a method for methylation of BTX aromatic hydrocarbons to synthesize mesitylene, CN 200910195550, application date 2009-09-11
[3] Ke Bocheng, Ke Boliu, Ke Baolai, Method for producing trimellitic anhydride by air segmented oxidation method, CN 200810122196, application date 2008-11-11
[4] Zeng Qingyu, Xia Xiaozhong, Liu Xianghong, a method for synthesizing trimethylhydroquinone from trimethylbenzene, CN 201611001125, application date 2016-11-15

 微信扫一扫打赏
微信扫一扫打赏

