Background and overview[1][2]
Bisphenol A diglycidyl ether (DGEBA) is a light yellow liquid epoxy compound formed by the condensation of epichlorohydrin (ECH) and bisphenol A (BPA). DGEBA can be used as an epoxy resin, and can also be used in coatings, adhesives, insulating paints, and packaging materials for electronic components such as semiconductors and integrated circuits. In recent years, DGEBA has been used in the inner coating of packaging materials such as canned food. However, chemical contaminants in the coating will migrate to the contents during canning processing and storage, thus contaminating canned food.
Apply[1-2]
In recent years, with the development of the electrical and electronic industries, the requirements for epoxy resins have become higher and higher, especially in the direction of refinement such as high purity, low viscosity, and low chloride ion content, such as those used in high-voltage motors. Pure epoxy anhydride type vacuum pressure impregnation (VPI) resin and high-purity, low-viscosity epoxy resin in electrical casting, electronic industry, and high-end light-emitting diode (LED) potting resin. Bisphenol A diglycidyl ether can be used as an epoxy resin, and can also be used in coatings, adhesives, insulating paints, packaging materials for electronic components such as semiconductors and integrated circuits. Such as two-component water-based epoxy coatings used to prepare aluminum alloy materials and composite materials. Due to the unique properties of aluminum alloy materials and composite materials, their application scope has been expanding year by year in recent years, and their usage has also increased.
In order to further improve the various resistance and decorative effects of the surfaces of aluminum alloy materials and composite materials, a relatively simple method is to apply paint on their surfaces. Both its component A and component B contain amine-terminated water-based amine-epoxy adduct, which is composed of 5 to 10% olefin-based polyamine, 5 to 12% monoepoxy compound, and 10 to 20% polyether polyol. %, bisphenol A diglycidyl ether 15~25%, organic acid 0~5%, co-solvent 15~20% and deionized water 25~40% as raw materials, prepared by step-by-step polymerization in the presence of a catalyst; components A is composed of amine-terminated water-based amine-epoxy adduct 30~50%, pigment 20~40%, filler 0~15%, defoamer 0.1~1%, wetting and dispersing agent 0.1~1%, deionization It consists of 15~30% water and 1~15% solvent; Component B is composed of 50~65% bisphenol A diglycidyl ether, 3~10% amine-terminated aqueous amine-epoxy adduct, and 25% deionized water. ~45% composition.
Hazards[3]
Bisphenol A diglycidyl ether (BADGE) is a member of the glycidyl ether family. After the 1940s, glycidyl ethers were widely used as basic materials for epoxy resin polymerization. In order to improve the storage life and corrosion resistance of food, the inner walls of metal food containers are generally coated with epoxy resin paint to prevent direct contact between the contents and the metal. Among them, BADGE will migrate to the contents during the processing and storage of food contact materials, causing contamination. The most commonly reported harm to human health caused by BADGE is sensitization reactions. There are reports that BADGE is an environmental hormone, also known as an exogenous endocrine disruptor, which can cause harm to human health even at extremely low intakes and endanger the ecological environment. In vitro studies have shown that BADGE has low estradiol receptor binding capacity. Whether the exogenous hormone activity of BADGE is other than itself or whether it may be metabolized into bisphenol A (BPA) in the body and interfere with endocrine function has always been a hot research topic. Investigations into workers handling epoxy resin revealed that BADGE can produce bisphenol A (BPA) in the body. During food processing, storage and transportation, BADGE will form hydrates, chlorinated compounds and other derivatives.
Preparation[1,4]
Method 1: Possible mechanism for synthesizing bisphenol A diglycidyl ether type epoxy resin: First, the phenolic hydroxyl group of BPA reacts with the epoxy group of ECH to form chlorohydroxyl ether, and then HCl is removed under the action of alkali to form Epoxide. In the presence of a base, BPA and ECH first undergo a ring-opening reaction and then close the ring. The synthesis route is shown in the figure:
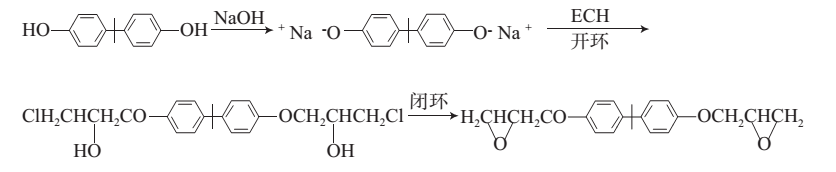
Add 1.237 g of BPA, 4.47 mL of ECH, 0.44 mL of water, and 3.44 mL of isopropyl alcohol to a four-necked flask equipped with a stirrer, dropping funnel, and reflux condenser. Stir to dissolve the BPA; heat to 75 ℃, slowly and uniformly add 2 mL of NaOH aqueous solution with a mass fraction of 30% under stirring, and finish the dripping in 45 to 50 minutes. Stir the reaction at constant temperature for 170 minutes; after the reaction is completed and cooled, add 50 mL of CHCl3 to the four-necked flask. Transfer the reaction solution to a separatory funnel and wash it three times with deionized water; separate the layers and separate.Transfer the � layer (organic layer) to a beaker, add anhydrous MgSO4, and stir for 1 h; filter to remove MgSO4, distill the filtrate on a rotary evaporator to remove the solvent, and then use silica gel column chromatography to separate and purify (using CH2Cl2 as the eluent ), distill the eluent on a rotary evaporator to obtain relatively pure DGEBA, which is a light yellow viscous liquid. Dry under vacuum for 24 hours to completely volatilize CH2Cl2; then place it in a cool, dry place for a period of time to obtain white crystals. Bisphenol A diglycidyl ether.
Method 2: A method for synthesizing bisphenol A diglycidyl ether by halogen-free epoxidation, including the following steps:
a. Ingredients: According to the molar ratio of bisphenol A diallyl ether:phosphotungstic acid quaternary ammonium salt catalyst to 1000:9~16, take bisphenol A diallyl ether and phosphotungstic heteropoly acid. For the quaternary ammonium salt catalyst, the added volume of solvent I is 2 to 6 times the added volume of bisphenol A diallyl ether. Take solvent I; the added molar amount of hydrogen peroxide is bisphenol A diallyl ether. Take 2 to 3 times the molar amount of hydrogen peroxide aqueous solution. The weight percentage concentration of the hydrogen peroxide aqueous solution is 15 to 70%; the solvent I is dichloromethane, methyl isobutyl ketone, chloroform, 1,2 -One or a mixture of two or more dichloroethanes;
b. Epoxidation reaction: Add bisphenol A diallyl ether, solvent I, and phosphotungstic heteropoly acid quaternary ammonium salt catalyst into the reactor, add hydrogen peroxide aqueous solution under stirring, and mix the reactants The temperature rises to 30-80°C and reacts at 30-80°C for 5-24 hours, that is, the reaction is completed and the reaction material is obtained;
c. Post-processing: Cool the reacted material to room temperature, separate the organic phase, distill the separated organic phase to recover the organic solvent, and stir the remaining material with the solvent Ⅱ ethyl acetate evenly (until soluble (dissolved), filter, and the solid obtained after filtration is the phosphotungstic heteropoly acid quaternary ammonium salt catalyst (which can be recycled and reused). The filtrate is then distilled to recover solvent II ethyl acetate to obtain the crude product; then use solvent III to The crude product is subjected to column chromatography to obtain bisphenol A diglycidyl ether;
Main reference materials
[1] Synthesis of bisphenol A diglycidyl ether
[2] CN200810195856.3 Two-component water-based epoxy coating for aluminum alloy materials and composite materials
[3] Ding Li, Gong Qiang, Zhu Shaohua, Jiao Yanna, Fu Shanshan, & Wang Libing. (2016). Study on the in vitro metabolism of bisphenol A diglycidyl ether, a harmful substance in food packaging materials. Journal of Food Safety and Quality Testing, ( 1), 102-107.
[4] CN201310160263.4 A method for halogen-free epoxidation synthesis of bisphenol A diglycidyl ether

 微信扫一扫打赏
微信扫一扫打赏

