Background
Phenylhydrazine hydrochloride can be used in the dye industry as a dye intermediate to produce naphthol AS-G; in the pharmaceutical industry as a raw material for synthetic drugs; in the pesticide industry as an intermediate for synthetic pesticides; in analytical chemistry as a test sugar Special reagents for ethers and ketones.
Synthesis【1】【2】
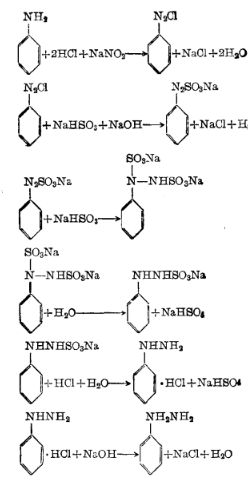
1. Using aniline diazo reduction acid precipitation method
First add water, 30% hydrochloric acid and aniline to the reaction pot, cool to 2°C, start adding sodium nitrite solution dropwise, control the reaction temperature to 0-5°C, stop adding materials until the potassium iodide starch test paper turns blue, and then continue Stir for 30 minutes to obtain diazo liquid and enter the reduction process.
First add water, sodium bisulfite and 30% liquid alkali to the reduction pot, raise the temperature to 80°C, add the above conditions at a pH value of 6.2-6.7 and a reaction temperature of 80-85°C. Diazo liquid, after stirring for 1.5 hours, add zinc powder and diatomite, and then stir and filter to obtain the filtrate, which is the sulfonated liquid, and then enter the acid precipitation process. Add the above sulfonation liquid and hydrochloric acid to the acid precipitation pot for acid precipitation. Control the acid precipitation temperature at 85-90°C. After the addition is completed, stir for another 10 minutes, then cool to below 20°C. After filtration, the filter cake is phenylhydrazine salt. Acid.
The chemical reaction equation:
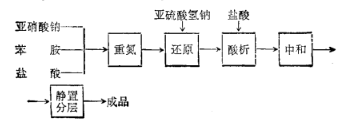
Process flow:
2. Using p-nitrobenzyl chloride as starting material
p-nitrobenzyl chloride (compound 2) is used as the starting material, and p-nitrobenzene methanesulfonyl chloride (compound 3) is prepared by sulfonation with sodium sulfite, chlorination with chlorine, and substitution with tetrahydropyrrole, and then treated with Fe2O3-MgO catalyst Compound 5 was obtained by reduction of hydrazine hydrate, and the target product (compound 1) was obtained through diazotization and reduction with insurance powder.
See the synthesis roadmap below:
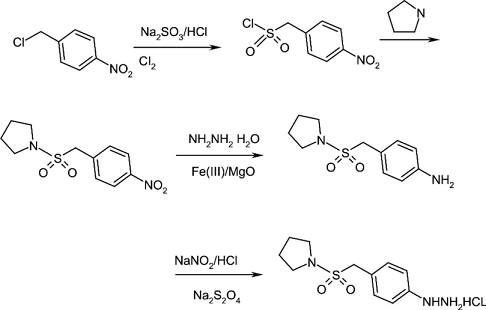
(1). p-nitrobenzenesulfonyl chloride (compound 3)
To a solution of sodium sulfite (14.0 g, 0.11 mol) and H2O (45 mL), compound 2 (17.2 g, 0.1 mol) was added under vigorous stirring.l) and methanol (30 mL) solution, heat to reflux for 2 h, then distill out the methanol, add water (100 mL), adjust the pH to 2 with concentrated hydrochloric acid, freeze to -5°C, slowly add chlorine gas, thin layer chromatography (TLC) Method (the developing agent is n-butanol) shows that after the raw materials are consumed, filter, wash with water, and wash with petroleum ether to obtain a light yellow solid. After drying, the solid can be directly used in the next reaction. Recrystallize from toluene to obtain compound 3 (21.1 g, 89.3%) as yellow needle crystals with a melting point (mp) of 90-91°C.
(2). p-Nitrophenylmethylsulfonylpyrrolidine (compound 4)
Add compound 3 (13.2 g, 0.06 mol) to dichloromethane (90 mL) with stirring. After it is dissolved, slowly add pyrrole (12 . 5 g, 0. 18 mol), control the reaction temperature not to exceed 12 ° C, return to room temperature after addition, react for 10 h, then wash with water (3 × 300 mL) until neutral, extract with dichloromethane, combine the organic phases, and dry After concentrating the organic solvent, a white solid compound 4 (12.4 g, 76.5%) was obtained.
(3). 4-(pyrrolidine-1-ylsulfonylmethyl)aniline (compound 5)
Dissolve Mg(NO3)2·6H2O (25.6 g, 0.1 mol) and Fe(NO3)3·9H2O (20.2 g, 0.05 mol) in water (80 mL), Add dropwise to a mixed solution of 2 mol/L NaOH and 0.1 mol/LNa2CO3 (150 mL) under strong stirring, raise the temperature to 85 °C and keep for 2 h, filter, wash with deionized water, dry at 60 °C for 24 h, and 450 °C Roast for 2 hours, then naturally cool to room temperature to prepare the Fe2O3-MgO hydrogenation catalyst, which is screened and set aside.
Add compound 4 (13. 5 g, 0. 05 mol) to a mixture prepared from 100 mL isopropyl alcohol and 50 mL tetrahydrofuran, and add 0.0 catalyst prepared by the above method. 40 g, add dropwise 80% hydrazine hydrate (7.4 g, 0.125 mol) at 70°C while stirring and maintaining slight boiling. After 30 minutes of addition, keep refluxing for 3 hours. TLC method (the developing solvent is n-hexane: Ethyl acetate = l ∶ 1) After the raw materials are shown to be consumed, filter while hot and wash the catalyst with a small amount of tetrahydrofuran. The catalyst can be reused (add 10% and can be reused 3 times). After distilling the solvent, a white solid compound 5 ( 11. 0 g, 92%).
(4). Phenylhydrazine hydrochloride (compound 1)
Add compound 5 (12.0 g, 0.05 mol) to concentrated hydrochloric acid (100 mL) and water (50 mL), heat to 45 ℃ and keep for 15 min, then cool to -5 ℃ in an ice-salt bath. Add 40% sodium nitrite (3.80 g, 0.055 mol) aqueous solution dropwise into the constant pressure dropping funnel, stir at the same temperature for 1 hour, and obtain the diazotization reaction solution for later use.
Dissolve insurance powder (30 g, 0.15 mol), water (100 mL), sodium hydroxide (4 g, 0.1 mol), and tetrahydrofuran (10 mL) and cool to 0°C. Follow the above method The prepared diazotization reaction solution was dropped into the insurance powder solution, keeping the reaction temperature below 5°C. After the addition, return to room temperature within 1 hour, heat to 80°C and keep for 1 hour, filter, add 100 mL of concentrated hydrochloric acid at 80 The reaction was maintained at ℃ until colorless, cooled to 0 ℃ in an ice-salt bath, a yellow solid precipitated, stirred for 25 min, and filtered with suction to obtain a yellow solid compound 1 (10.0 g), with a yield of 68.6%.
Packaging, storage, transportation and hazardous characteristics【1】
The packaging should have a clear “drug-containing” sign. It is an organic drug-containing product, and the dangerous regulation number is 84224. Store in a cool, ventilated, dry warehouse, away from fire and heat sources, away from sunlight and heat, and stored separately from oxidants, acids and food additives. When handling, load and unload with care to prevent damage to the packaging, and store and transport according to regulations for toxic chemicals.
This product is toxic. Regular contact will damage its main functions and cause chronic poisoning. It can also cause allergies to the eyes, respiratory tract and skin. The maximum allowable concentration in the air is 55ppm, which requires the production equipment to be sealed, the workshop to be well ventilated, and the staff to wear labor protection equipment. This crystal is flammable and can burn when exposed to open fire. When heated and decomposed, it will release toxic nitrogen gas and react violently with oxidants and lead dioxide. In case of fire, use foam, carbon dioxide, powder, sand, and mist water to extinguish the fire.
Toxicity【3】
Phenylhydrazine hydrochloride is the main intermediate for the synthesis of oil-soluble colorants and the raw material for pharmaceuticals. There have been some reports on the toxic effects of phenylhydrazine hydrochloride. It mainly manifests as sensitization to the skin and can cause acute and chronic eczema. It also causes varying degrees of damage to the liver, kidneys, nervous system, and blood system, and can cause hemolytic anemia, hyperbilirubinemia, urobilinogen, methemoglobin, Hern’s bodies, hepatomegaly, and hepatomegaly. Abnormal performance. Mild cases include headache, pale complexion, loss of appetite, abdominal pain and diarrhea; severe cases include dizziness, shortness of breath, methemoglobinemia, hemolytic anemia, hematuria, etc. Therefore, it is imperative to reduce the exposure concentration, reduce toxic reactions, and reform the process flow.
References
[1] Chen Songmao, Practical Handbook of Chemical Products (3), Shanghai Science and Technology Literature Press, 1990.10, page 361
[2] Xia Kejian, Preparation of (pyrrolidin-1-ylsulfonylmethyl)phenylhydrazine hydrochloride, China Pharmaceutical Industry, 2014.08, page 35
[3] Xue Shougui, Chen Xueshi, Liang Shangmin, Modern Clinical Medicine Research and Practice Volume 1, China Science and Technology Press, April 1993, 1st edition, page 623

 微信扫一扫打赏
微信扫一扫打赏

