Background and overview[1][2]
4-Methoxytriphenylmethane chloride is mainly used as a pharmaceutical and chemical intermediate for the synthesis of other substances. If 4-methoxytriphenylmethane is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
Apply[2-3]
4-Methoxytriphenylmethane chloride is mainly used as a pharmaceutical and chemical intermediate for the synthesis of other substances. Examples of its applications are as follows:
1. Synthesize entecavir as an intermediate.
Entecavir, also known as entecavir hydrate and entecavir monohydrate, is the latest first-line drug against hepatitis B virus. Entecavir is a cyclopentylguanosine analogue. Phase II/III clinical studies have shown that adults taking 0.5 mg orally per day can effectively inhibit HBV DNA replication, and the efficacy is better than lamivudine; Phase III clinical studies have shown that increasing the dose to 1 mg daily can be effective for patients with YMDD mutations. Inhibits HBV DNA replication. The incidence of drug resistance in first-year patients was 0, but in patients with YMDD mutations, the incidence of drug resistance in patients with YMDD mutations was 5.8%. my country’s SFDA has also approved it for the treatment of patients with chronic hepatitis B. 4 Methoxytriphenylmethane is one of the intermediates in the synthesis of entecavir. The specific steps are as follows:
1) In a nitrogen atmosphere, add Zn to tetrahydrofuran, cool to -40°C, add CH2Br2, keep warm, and add TiCl4 while stirring. Naturally raise the temperature to 5°C and keep the temperature for 4 days to obtain NYSted reagent;
2) Under nitrogen protection, add (2R,3S,5S)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6- into the reactor at room temperature. Benzyloxy-9H-purin-9-yl]-3-benzyloxy-2-[(benzyloxy)methyl]cyclopentanone and anhydrous CHCl3, then add anhydrous Stir triethylamine, Li, 4-methoxytriphenylmethane chloride and dimethylaminopyridine, react in the dark at 20°C for 24 hours, and perform silica gel column chromatography to obtain Ente-8;
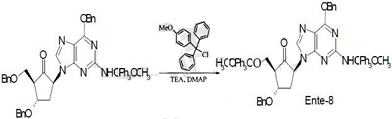
3) Under nitrogen atmosphere, add Ente-8 and CH2Cl2 to the reaction tank, stir, add NYSted reagent, stir at room temperature for 5 hours, add the reaction solution to the mixed solution of saturated sodium bicarbonate and chloroform, and stir for 30 minutes , centrifuge, separate the liquids, extract the aqueous layer three times with chloroform, combine the organic layers, wash three times with saturated sodium chloride solution, dry with anhydrous sodium sulfate, filter, and rotary evaporate to remove the solvent to obtain a residue, which is purified by column chromatography to obtain Ente- 9;
4) Add THF, methanol and Ente-9 to the reaction flask, stir, add HCl, heat to 50°C, react for 1 hour. After the reaction is completed, lower to room temperature, adjust the pH value to 7 with NaOH, and use ethyl acetate Extract the ester 3 times, combine the organic layers, wash 3 times with saturated sodium chloride solution, dry with anhydrous sodium sulfate, filter, rotary evaporate to remove the solvent to obtain the residue, beat with ether to obtain Ente-10; 5) Under nitrogen atmosphere, add to Add Ente-10 and methylene chloride to the reaction tank, stir, cool down to -80°C, add BCl3 dichloromethane solution dropwise, raise the temperature to -40°C, react for 3 hours. After the reaction is completed, add methanol dropwise, and evaporate to remove the solvent. Obtain crude Entecavir, refine it, and prepare pure product.
2. Used to synthesize 4-methoxytriphenylmethyl-protected aminophosphoramidite monomer,
The specific steps are as follows:
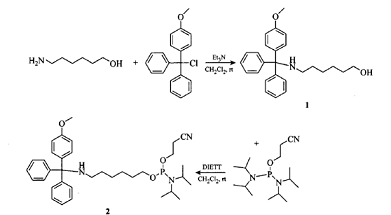
Step 1: Add 6-amino-1-hexanol (58.6 g, 0.5 mol) and 800 ml dichloromethane into a three-necked flask in sequence, stir and dissolve, then add 104.5 ml triethylamine (75.9 g, 0.75 mol) , 1 mol/I4-methoxytriphenylmethane chloride solution in dichloromethane (200.0 g, 0.65mol, 650 mL). After the dropwise addition of 4-methoxytriphenylmethane is completed, let the reaction naturally rise to room temperature. Continue stirring for 4 h and then add 500 mL water to quench the reaction. After liquid separation, add 500 mL of saturated NaHCO3 solution to the lower layer, shake, let stand, and separate layers. Collect the lower organic phase and dry it over anhydrous sodium sulfate. After suction filtration, the filtrate is concentrated. After purification by column chromatography, compound 1 (144.2 g) is obtained. ), the yield is 74.0%.
Step 2: Place compound 1 (39.0 g, 0.1 mol) in a flask, add 300 mL of dichloromethane, stir and dissolve. Add 7.0 g coupling agent (1,2,4-triazole:diisopropylamine-2:1) and phosphorus reagent (45.2 g, 0.15 mol) at room temperature in sequence, and stir at 25°C for 3 h. Add 200 ml saturated NaHCO3 for liquid separation, then transfer the lower layer to a separatory funnel, add 200 ml water, shake, let stand, separate layers, collect the lower organic phase, and discard the upper layer. Then transfer the lower layer to a separatory funnel, add 200 ml saturated NaCl, shake, let stand, separate layers, collect the lower organic phase, and dry over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated and purified by column chromatography to obtain 51.3 g of aminophosphoramidite monomer (5,1_Amino-Modifier C6-MMT), with a yield of 86.9%.
Preparation [1]
A method for synthesizing 4-methoxytriphenylmethane chloride, the synthesis path is as follows:
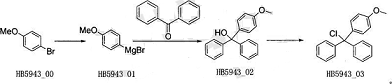
Add 21.2g (883.33mmol, 1.1eq) magnesium, 240mL absolute anhydrous tetrahydrofuran and 5 grains of iodine into a 1L four-necked flask under nitrogen protection; then add 150g (802.02mmol, 1eq) HB5943_00 and 250mL absolute anhydrous tetrahydrofuran One-tenth of the mixed solution was added to the reaction solution and began to be slowly heated. When the heating started to reflux, the Grignard reaction was initiated and the color of the solution faded. Then, while maintaining reflux, add the remaining nine-tenths of the mixed liquid dropwise to the reaction system. After the dropwise addition is completed, reflux for 3 to 4 hours.
Cool the reaction solution to below 20°C, and then add a mixture of 131.5g (721.81mmol, 0.9eq) benzophenone and 200mL absolutely anhydrous tetrahydrofuran dropwise into the reaction solution. There will be an exothermic phenomenon during the dropwise addition. Control the internal temperature below 30°C and reflux for 2 to 3 hours after the dripping is completed. The reaction solution was quenched with 400 mL of saturated ammonium chloride, and the organic layer was separated. The aqueous layer was extracted with ethyl acetate (300 mL*3). The organic phases were combined, dried over anhydrous sodium sulfate, and spun to dryness to obtain an oily substance. The oil was heated and dissolved with a mixture of ethyl acetate/petroleum ether = 1/4, and then cooled and stirred in an ice-salt bath. A large amount of white solid precipitated out, and was filtered to obtain a white solid.
Main reference materials
[1] Liu Jinquan. Research on the synthesis process of amino-modified phosphoramidite monomers[J]. Chemistry World, 2014, 55(10): 609-611.
[2] CN201210093813.0 Synthesis method of 4-methoxytriphenylmethane chloride

 微信扫一扫打赏
微信扫一扫打赏

