Background and overview[1][2]
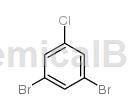
The Chinese aliases of 1,3-dibromo-5-chlorobenzene are 1-chloro-3,5-dibromobenzene and 3,5-dibromochlorobenzene, CAS number 14862-52-3, chemical formula C 6H3Br2Cl. Molecular weight 270.34900. White to light yellow crystal powder, density 2.021 g/cm3, melting point 91-94 °C (lit.), boiling point 256 °C, flash point 115.8ºC, refractive index -38 ° (C=0.1, H2O), vapor pressure 0.0356 mmHg at 25°C. 1,3-Dibromo-5-chlorobenzene is an important chemical raw material and pharmaceutical intermediate. 1,3-Dibromo-5-chlorobenzene is a reactant for the preparation of orally bioavailable 1-(1H-indol-4-yl)-3,5-disubstituted benzene analogues as antimitotic agents. If 1,3-dibromo-5-chlorobenzene is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
Application[1, 3]
1,3-Dibromo-5-chlorobenzene is a reaction for the preparation of orally bioavailable 1-(1H-indol-4-yl)-3,5-disubstituted benzene analogues as antimitotic agents things. In addition, 1,3-dibromo-5-chlorobenzene can also be used to prepare compounds:
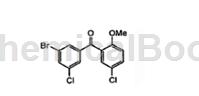
The reaction steps are as follows: A solution of 1,3-dibromo-5-chlorobenzene (7.62g, 28.2mmol) in 100mL diethyl ether is cooled to -78°C in a dry ice/acetone bath. Add n-butyllithium (12.6 mL of 2.5 M in hexanes, 31.5 mmol) dropwise over 30 minutes. The resulting mixture was stirred at -78 °C for a further 13 min, then 183 (6.57 g, 28.6 mmol) was added in small portions over 23 min. The reaction mixture was then stirred for 22 hours while the bath temperature was raised to room temperature. The mixture was poured into 100 mL of water and extracted with two 100 mL portions of EtOAc. The combined organic layers were then dried over MgSO4, filtered, and concentrated in vacuo to give 9.46 g of beige solid. Recrystallization from hot MeOH gave the compound of the above structure.
Preparation [2, 4-5]
A solution of 1,3,5-tribromobenzene (9.44g, 30mmol) in 120mL of diethyl ether was cooled to -78°C in a dry ice/acetone bath. n-Butyllithium (13.2 mL of 2.5 M in hexanes, 33 mmol) was added dropwise over 10 min. The resulting mixture was stirred at -78 °C for a further 10 min, then hexachloroethane (7.15 g, 30.2 mmol) was added in small portions over 3 min. The reaction mixture was then stirred at -78°C for 15 minutes and then at room temperature for 3.2 hours. The mixture was partitioned between 100 mL water and 100 mL EtOAc. The aqueous layer was separated and extracted with an additional 100 mL of EtOAc. The combined organic layers were then dried over MgSO, filtered, and concentrated in vacuo to afford 1,3-dibromo-5-chlorobenzene as a light brown solid (7.72 g, 95%).

 微信扫一扫打赏
微信扫一扫打赏

