Background and overview[1]
3-[[3-[(Dimethylamino)carbonyl]-2-hydroxyphenyl]amino]-4-[[(R)-1-(5-methylfuran-2-yl)propan [Hydroxy]amino]-3-cyclobutene-1,2-dione, also known as 2-hydroxy-N,N-dimethyl-3-[[2-[[1(R)-[5-methyl -4-(1-methylethyl)-2-furyl]propyl]amino]-3,4-dioxo-1-cyclobuten-1-yl]amino]-benzamide, is a type of 1,2-substituted 3,4-dioxo-1-cyclobutene compounds. It is a potent allosteric antagonist of CXCR1 and CXCR2 and is for scientific use only.
Preparation[1]
2-Hydroxy-N,N-dimethyl-3-[[2-[[1(R)-(5-methyl-2-furyl)propyl]amino]-3,4-di Preparation of oxy-1-cyclobuten-1-yl]amino]benzamide monohydrate (Type 4):
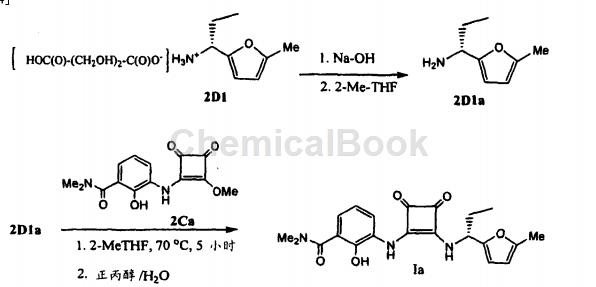
To a suspension of 10.1 g (2D1) (1.06 equivalents) in 30 ml water and 40 ml 2-methyltetrahydrofuran, add 6.5 ml 32% sodium hydroxide solution. Test the formed water layer with pH paper. If the pH is below 13, add another small amount of caustic solution. The organic layer was separated, and the aqueous layer was extracted with 20 ml of 2-methyltetrahydrofuran. The combined organic layers were mixed with 10.0 g (1.0 equiv) (2C) and the suspension was heated at 70°C for 5 hours until less than 1.0% of starting material remained. n-Propanol (50ml) was added. The volume of the reaction mixture was reduced to 40 ml (4X) by partial vacuum distillation, followed by the addition of a further 50 ml of n-propanol. The volume of the solution was then reduced to 60 ml under partial vacuum.
Dilute the mixture to 90 ml with n-propanol and add 0.3 ml of acetic acid. Then filter the solution. The filtrate was then diluted with 140 ml n-propanol, and the solution was heated to 70°C. Water (125ml) was added while maintaining the batch temperature above 70°C. The solution was cooled to 62°C and 200 mg of compound seed crystals were added. The mixture was stirred at 62°C for 2 hours and then cooled to 20°C over approximately 5 hours. The suspension was then heated to 55°C over 30 minutes and then slowly cooled to 20°C over 4 hours. The heating and cooling operations are repeated several times to grow crystals of the desired particle size. The suspension was finally cooled to 20°C before filtration. The wet cake was washed with 80 ml of a solvent mixture of n-propanol and water (1:1).
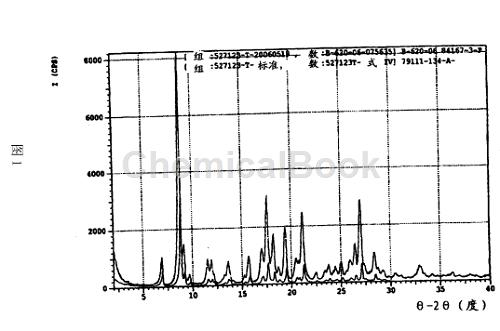
Dry the filter cake at 50°C for 12 hours or until KF analysis shows the moisture content is less than 4.7%, yielding 11.5g (85%) of white needle-like crystals, m.p. 83°C. 1HNMR(DMSO-D6)δ, 0.91(t, 3H, J=7.3), 1.84(m, 1H), 1.94(m, 1H), 2.25( s, 3H), 2.92 (S, 6H), 5.13 (m, 1H), 6.01 (d, 1H, J=3.1), 6.25 (d, 1H, J=3.1), 6.85 (m, 2H), 7.78 ( d, 1H, J=7.3), 8.65 (d, 1H, J=8.9), 9.29 (br, 1H), 9.99 (br, 1H). 13C NMR (DMSO-D6): 10.26, 13.32, 27.18, 52.78, 106.42, 107.52, 119.77, 120.76, 122.18, 124.42, 128.64, 143.25, 151.31, 152.06, 163.41, 168.27, 168.52, 180.17, 183.95, 184.71. Anal. Calcd. for C12H25N3O6 (monohydrate 415.4): C, 60.71; H, 6.07; N, 10.11. Actual measured values: C.60.65; H, 5.93; N, 9.91. Figure 1 represents the characteristic X-ray powder diffraction pattern of the compound of formula I, form IV [vertical axis; intensity CPS, counts (square root)), horizontal axis: 2θ (degrees)].
��Need reference materials
[1] CN200880103057.7 Method for controlling crystal size in 1,2-substituted 3,4-dioxo-1-cyclobutene compounds

 微信扫一扫打赏
微信扫一扫打赏

