Background and overview[1]
Tetrachlorophthalic anhydride, also known as tetrachlorophthalic anhydride or tetrachlorophthalic anhydride (TCPA), is a reactive flame retardant, widely used in unsaturated polyester, epoxy resin, polyolefin, film In synthetic materials such as film, it is also used in medicine, pesticides, military industry, and textiles. Tetrachlorophthalic anhydride has good compatibility with resin, high flame retardant efficiency, low cost, and does not change the resin properties. After adding tetrachlorophthalic anhydride flame retardant to synthetic materials, a decomposition reaction will occur when exposed to fire and heat. The decomposed chlorine free radicals react with synthetic materials to produce HCl. The reaction between HCl and HO– reduces the concentration of highly active free HO– and slows down the burning rate until The flame goes out. Therefore, tetrachlorophthalic anhydride is a promising reactive flame retardant.
Synthesis method[2][3][4][5]
Synthetic method 1: Tetrachloro-o-xylene oxidation and dehydration method
Use oxidizing agent to oxidize chlorotoluene to prepare tetrachlorophthalic anhydride: put 50g of 3,4,5,6-tetrachloroxylene, 500g of chlorosulfonic acid, 1.5g of iodine into a high-temperature kettle, and use vanadium-containing, Titanium and tungsten oxide catalysts are used for catalytic reaction, the temperature is controlled at 360~400℃, 3,4,5,6-tetrachlorotoluene is oxidized in the gas phase, and the space velocity is maintained at 4800~8400s-1. The catalyst can be prepared by sintering V2O5-TiO2-WO3. Its mass ratio is (87 ~92): (5~12): (1~3).
Synthesis method 2: Direct chlorination of phthalic anhydride
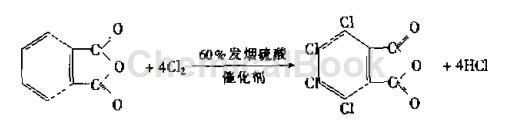
The direct chlorination method of phthalic anhydride can be divided into (1) the solvent method of synthesizing tetrachlorophthalic anhydride using iodine or iodine chloride as the catalyst and fuming sulfuric acid or chlorosulfonic acid as the solvent according to different chlorination conditions; (2) A melting method to prepare tetrachlorophthalic anhydride using Mg, Fe, Sb, etc. as catalyst, and chlorine gas is introduced into the molten phthalic anhydride; (3) FeCl3, CoCl2, etc. Lewis acid is used as a catalyst, and phthalic anhydride is synthesized by gas phase chlorination at high temperature (200~400℃). The preparation of tetrachlorophthalic anhydride by chlorination of phthalic anhydride is an electrophilic substitution reaction on the benzene ring. The reaction equation is shown in the figure below. During preparation, phthalic anhydride and fuming sulfuric acid are put into a reactor with a stirrer and a condenser at a certain molar ratio, a catalyst is added, stirring is started, heating is raised, and cooling water is turned on. When the phthalic anhydride is completely dissolved, dry chlorine gas is introduced. , the reaction releases hydrogen chloride gas, which becomes hydrochloric acid solution after being absorbed by water. Finally, the reaction temperature was controlled to 150°C, and the reaction was completed after 16 hours. Put the reaction solution into a crystallizer while it is hot and allow it to cool and crystallize naturally. After filtration, crude tetrachlorophthalic anhydride is obtained. The mother liquor can be recycled and weighed after refining, and the yield is stable at more than 80%. The process flow is shown in the figure below.

Application areas[6][7][8][9]
1.Synthetic dyes
Solvent Red EG (C.I. Solvent Red 135) is a benzene-type functional solvent dye. It is the most important dye variety among the benzone-type solvent dyes. It has excellent weather resistance and light resistance, excellent heat resistance, and bright colors. Pure, widely used in transparent or opaque coloring of various thermoplastic plastics, such as polystyrene, ABS resin, polymethyl methacrylate resin, acetate fiber and polyvinyl chloride, etc. It can also be used for polyester fiber and polyamide fiber. of puree coloring. The reaction formula for synthesizing solvent red EG from tetrachlorophthalic anhydride is shown in the figure below. Using tetrachlorophthalic anhydride and 1,8-diaminonaphthalene as raw materials, o-dichlorobenzene as the reaction solvent and glacial acetic acid as the catalyst, the preferred process conditions are 26.5g of tetrachlorophthalic anhydride and 1,8-diaminonaphthalene. 15g naphthalene, 235g o-dichlorobenzene, 5g glacial acetic acid; stir and react at 200 rpm at 115°C for 4 hours. The product synthesis yield is 98.03%. After the sample was colored with polystyrene, it was injection molded into sheets. The measured color difference was 0.18 compared to the high-quality standard product, and the coloring rate was 99.87%.

2. Used as flame retardant
Tetrachlorophthalic anhydride is a flame retardant material with excellent performance. It can significantly modify the combustion properties of flammable substances and increase the applicability and application value of the product. For example, a new flame-retardant unsaturated polyester resin is synthesized using tetrachlorophthalic anhydride as the reactive flame retardant and halogenated phosphate as the additive flame retardant. The process is shown in the figure below. The specific steps are: add glycol to the polycondensation kettle, heat the liquid components to about 100°C, start the stirrer, pass inert gas nitrogen into the alcohol, and add tetrachlorophthalic anhydride, fumaric acid, and phthalate in sequence. For solid materials such as formic anhydride, add 0.01-0.02% polymerization inhibitor, continue stirring and raise the temperature to 190~200°C, then maintain the temperature. During this period, the reaction proceeds very quickly and a large amount of water is generated. Open the fractionating column condenser. Control the distillation temperature between 100 and 105°C to reflux the alcohol, and open the cooling water valve of the recovery condenser to recover the reaction water and hydroalcohol until the end of the reaction. Cool the cold water through the snake-shaped tube in the kettle to 160°C and add paraffin. When the filler is ready, put the material into the dilution kettle, cool it to 85°C, add styrene cross-linking, and add BCP. The finished product is ready when the temperature is lowered to 40℃. All properties of the modified high flame retardant resin meet the national standard requirements.

3.Synthetic pharmaceutical intermediates
2,3,4,5-tetrafluorobenzoic acid is an important intermediate in the synthesis of new fluoroquinolone antibacterial drugs.It is used for the synthesis of quinolone drugs lomefloxacin, ofloxacin, rofloxacin, sefloxacin, etc. This intermediate can be prepared from tetrachlorophthalic anhydride, and its reaction formula is as follows. It mainly synthesizes tetrafluorobenzoic acid through four steps of imidization, fluorination, hydrolysis and decarboxylation.

Main reference materials
[1] Zhang Heng. Several important organochlorine flame retardants[J]. China Chlor-Alkali, 2002(7):25-27.
[2] SU 1719401
[3] Zhang Yue, Liu Haixia, Yang Mu. Preparation of tetrachlorophthalic anhydride [J]. Chemical Propellants and Polymer Materials, 1999(5):23-24.
[4] Wang Weijian, Xu Xuebo. Synthesis of tetrachlorophthalic anhydride[J]. New Chemical Materials, 2000(5):21-22.
[5] Zhang Yajing, Yang Jianping, Ye Bin, et al. Improvement of the solvent method for synthesizing tetrachlorophthalic anhydride [J]. Progress in Fine Petrochemicals, 2001, 2(5):1-3.
[6] Zuo Xinju, Xu Dexian. Research on the synthesis process of solvent red EG[J]. Dyes and Dyeing, 2011, 48(4):1-4.
[7] Yao Bo, Yu Xiuying. Engineering research on tetrachlorophthalic anhydride flame-retardant high-strength unsaturated polyester resin [J]. Heilongjiang Science and Technology Information, 2002(9):57-57.
[8] Cai Xingwei, Zhao Yuyuan, Jiang Dawei, et al. Synthesis and process optimization of 2,3,4,5-tetrafluorobenzoic acid[J]. Fine Petrochemicals, 2012, 29(3):52-54 .
[9] Fu Chun. Synthesis and application of 2,3,4,5-tetrafluorobenzoic acid[J]. Fine and Specialty Chemicals, 2001, 9(21):18-21.

 微信扫一扫打赏
微信扫一扫打赏

