Background and overview[1][2]
Meta-chlorobenzaldehyde is mainly used as an intermediate in organic synthesis of new pesticides, medicines, etc. It is the most expensive of the three isomers of monochlorobenzaldehyde. The current price of this product on the market is about 3 to 6 times higher than the other two isomers. Its profit It is also relatively high. Moreover, because domestic production processes and technologies are relatively backward, there is still a lot of room to reduce its raw material and production costs. It is a high value-added fine chemical product. Therefore, further reducing the production cost of m-chlorobenzaldehyde can bring greater benefits. great economic benefits.
Organic electrosynthesis can solve the above problems. This new organic synthesis method has developed rapidly in recent years. The electrochemical synthesis of ortho- and para-benzaldehyde has been reported in the literature. Some studies have used indirect electrooxidation method, using manganese (Ⅲ) in sulfuric acid medium as the oxidation medium to oxidize m-chlorotoluene to m-chlorobenzaldehyde. Electrolysis of Mn 2+ /Mn 3+ has high current efficiency in sulfuric acid medium. This method has a single product, high reaction selectivity, and the oxidation medium can be recycled. It has the advantages of simple process, low investment, high product purity, and no pollution. , so it is easy to realize industrialization and has broad development prospects.
Apply[3]
Meta-chlorobenzaldehyde is mainly used as an intermediate in organic synthesis of new pesticides, medicines, etc. For example, m-chlorobenzoic acid was synthesized from the oxidation of m-chlorobenzaldehyde. Meta-chlorobenzoic acid is an aromatic acid compound with colorless needle-like or monoclinic crystals and a melting point of 158°C. It is an important chemical intermediate. It is mainly used as a pharmaceutical intermediate for the synthesis of the antidepressant drug hydrochloric acid. Alpha-bromo-m-chloropropiophenone, the key intermediate of bupropion, can also be used as a fine chemical raw material for the synthesis of fine chemical products containing m-chlorobenzene ring structures in the molecules, and can also be used as an intermediate for other pharmaceuticals and pesticides. wait.
In acetic acid solution, m-chlorobenzaldehyde and potassium bromate were used as raw materials to synthesize m-chlorobenzoic acid. The effects of reaction temperature, reaction time, raw material ratio, solvent dosage and other conditions on the synthesis reaction were studied, and the optimal solution was determined. Best process conditions. The most suitable operating conditions for synthesizing m-chlorobenzoic acid by this method are: the reaction temperature is 90 ℃, the reaction time is 90 min, n (m-chlorobenzaldehyde): n (potassium bromate) = 1:0.45, and the solvent dosage is 40 mL ( Relative to 0.1 mol m-chlorobenzaldehyde). The yield of m-chlorobenzoic acid can reach more than 99.36%, and the purity of m-chlorobenzoic acid product w(m-chlorobenzoic acid) = 99.5%.
Preparation [4-5]
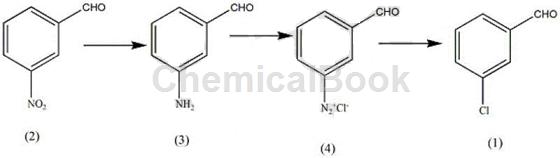
Method 1: A method for synthesizing the plant growth regulator indole drug intermediate m-chlorobenzaldehyde, including the following steps:
(i) In a reactor with a volume of 3-10L equipped with a stirrer, add a certain amount of 3-5 mol of cadmium halide crystals and 700-900 mL of acidic solvent, and stir to dissolve the cadmium halide crystals. , keep the solution at 2-4°C, add 0.7-1.2mol of o-nitrobenzaldehyde (2), maintain the reaction time for 10-15min, maintain the stirring speed at 800-1200rpm, and maintain the solution temperature at 80–90°C , after o-nitrobenzaldehyde is completely dissolved, the solution temperature drops to 3-5°C, orange-red crystals precipitate, and finally paste (3) is obtained; the cadmium halide can be cadmium chloride, bromide Any one of cadmium and cadmium iodide; the acidic solvent can be any one of phosphoric acid and phosphorous acid;
(ii) Dissolve a certain amount of nitrite in 160-200ml of aqueous solution, use a dropping funnel to drop the nitrite solution into the paste (3) obtained in step (i), and maintain the reaction temperature at 6–10°C, use a dropping funnel to complete the dropwise addition of the nitrite solution within 60 minutes, measure the reaction endpoint with potassium iodide test paper, and generate diazonium salt (4); the nitrite can be potassium nitrite , any one of magnesium nitrite;
(iii) Add a certain amount of nitrate and halide to 700-800ml aqueous solution, maintain the temperature of the aqueous solution at 80-90°C, add a certain amount of phosphite, and add alkali to the aqueous solution to form a solution, and heat The solution reaches 60°C to generate a halide salt solution; add the diazonium salt solution to the hot halide salt solution, maintain the stirring speed at 900 rpm for 8-10 minutes, add 900-1000 mL of acid solution, and let it stand 15-26h, distill with water vapor, extract the distillate with an organic solvent, distill the solvent and then distill under reduced pressure, collect the fractions at 90–95°C to obtain m-chlorobenzaldehyde (1).
The nitrate can be any one of sodium nitrate, potassium nitrate, and ferric nitrate; the halide can be any one of sodium bromide, potassium bromide, and magnesium bromide; The phosphite can be any one of iron phosphite and magnesium phosphite; the alkali is any one of sodium carbonate and potassium carbonate; the acid solution is hypochlorous acid; the organic solvent is methyl ether , benzene, and methanol; the ratio of the amounts of o-nitrobenzaldehyde and nitrate is 1:1-1.3, and the entire reaction process can be expressed by the following reaction formula:
Method 2: A method of continuously oxidizing m-chlorotoluene to prepare m-chlorobenzaldehyde using a tubular reactor with a special structure. Follow the following steps:
(1) First, at room temperature, stir and mix the substrate m-chlorotoluene and part of the carboxylic acid solvent in a volume ratio of 1:1, mix the oxidant and part of the carboxylic acid solvent in a volume ratio of 1:1, and then The metal complex is mixed and poured into the m-chlorotoluene-carboxylic acid solution, and the sodium salt is poured into the hydrogen peroxide-carboxylic acid solution; through the required reaction time, the different flow rates of the two materials are calculated, and the different flow rates of the two materials are continuously pumped through the metering pump. It enters the tubular reactor and is preheated and mixed before entering the reaction zone for reaction, and the reaction temperature is controlled by an external circulation heat exchange system;
(2) Control the molar ratio of the reaction materials by adjusting the flow rate and weighting, and control the residence time of the material mixing reaction from 60 to 1800s by changing the inner diameter of the pipe of the tubular reactor from 0.5 to 15 mm and the volume from 25 to 750 ml; After the reaction is completed, the product flows out from the end of the reactor and enters the collection tank. The product is distilled and separated. The unreacted m-chlorotoluene is recycled and reacted. The product m-chlorobenzaldehyde is collected after distillation and purification. The target product m-chlorobenzaldehyde is The yield can reach 20% to 40%.
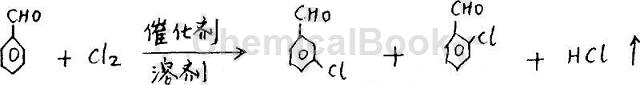
Method 3: In a three-necked flask device equipped with a stirrer, first add 600 grams of dichloroethane, while stirring, add 300 grams of aluminum trichloride, and then heat to raise the temperature in the kettle to 55°C, and Keep the temperature in the kettle at 55-60°C, add 184 grams of benzaldehyde dropwise, continue stirring for 20 minutes, lower the temperature in the kettle to 39-40°C, pass in 120 grams of chlorine, stop passing chlorine, and hydrolyze the chlorinated liquid. Send it to the rectification tower for fractionation to obtain 128.8 grams of m-chlorobenzaldehyde (content reaches 98%), 14.70 grams of o-chlorobenzaldehyde (content reaches ≥95%), and 35 grams of reacted benzaldehyde. The selectivity is 75% and the reaction conversion rate is over 90%. Its chemical reaction formula is:
Main reference materials
[1] CN201610972043.5 A method for preparing m-chlorobenzaldehyde by continuous oxidation of m-chlorotoluene
[2] Synthesis of m-chlorobenzaldehyde by indirect electrooxidation method
[3] Research on the synthesis of m-chlorobenzoic acid from the oxidation of m-chlorobenzaldehyde
[4] CN201510979925.X A synthesis method of plant growth regulator indole drug intermediate m-chlorobenzaldehyde
[5] CN97104185.7 Method for producing m-chlorobenzaldehyde from benzaldehyde

 微信扫一扫打赏
微信扫一扫打赏

